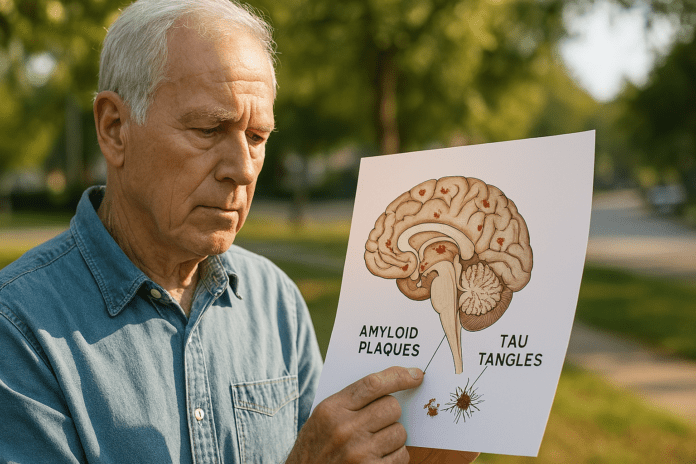
Alzheimer’s disease, one of the most prevalent neurodegenerative disorders globally, affects millions of people and places a substantial burden on individuals, families, and healthcare systems. Central to the pathology of Alzheimer’s are two protein abnormalities: amyloid plaques and neurofibrillary tangles composed of tau protein. These biological hallmarks serve not only as diagnostic indicators but also as key contributors to disease progression. Over the last few decades, scientists have extensively investigated how amyloid beta and tau proteins interact to form these plaques and tangles in the brain, attempting to untangle their roles in memory loss, cognitive decline, and neuronal dysfunction. While our understanding of Alzheimer’s continues to evolve, much of the conversation surrounding the disease hinges on decoding what causes amyloid plaques, how tau tangles develop, and the implications of these abnormalities on brain health.
You may also like: How to Prevent Dementia and Alzheimer’s Disease Naturally: Expert-Backed Strategies to Reduce Your Risk Through Lifestyle and Diet
The Structure and Function of Amyloid Beta in the Brain
Amyloid beta is a protein fragment derived from the larger amyloid precursor protein (APP), which is normally involved in neuronal growth and repair. Under healthy conditions, enzymes process APP into soluble amyloid beta peptides that are cleared from the brain. However, in Alzheimer’s disease, this clearance becomes impaired. The peptides begin to accumulate and aggregate, forming insoluble beta amyloid plaques. These amyloid plaques, often found between neurons, are a defining feature of amyloid Alzheimer pathology.
Research has shown that beta amyloid plaques can disrupt cell-to-cell communication and activate immune responses that cause chronic inflammation and neuronal death. This process is thought to contribute significantly to cognitive symptoms such as memory loss and confusion. The accumulation of amyloids in the brain is not merely incidental; it reflects a broader dysfunction in protein regulation and synaptic maintenance. Scientists continue to explore why the body fails to eliminate these proteins effectively and what causes plaque in brain tissue to reach neurotoxic thresholds.
The presence of b amyloid plaques alone does not always correlate with the severity of Alzheimer’s symptoms, indicating that other factors, including tau tangles and neuroinflammation, play essential roles. Nonetheless, amyloid beta and tau together are now widely recognized as critical components of Alzheimer’s disease development, with their interplay increasingly considered in both diagnostic criteria and treatment research.
The Origin and Impact of Tau Neurofibrillary Tangles
Tau is a stabilizing protein primarily found in neurons where it supports the microtubules that serve as internal scaffolding for cellular transport. In healthy brains, tau undergoes phosphorylation, a chemical process that helps regulate its function. However, in Alzheimer’s disease, tau becomes excessively phosphorylated and begins to misfold, detaching from microtubules and aggregating into twisted strands known as tau neurofibrillary tangles. These tangles are found inside neurons and are particularly damaging because they interfere with the cell’s transport system and eventually lead to cell death.
The presence of tau tangles has a strong association with the progression and severity of Alzheimer’s symptoms. Unlike amyloid plaques, which tend to form early and in broader cortical regions, tau pathology follows a more predictable pattern, beginning in the entorhinal cortex and hippocampus—regions critical for memory—before spreading throughout the brain. As such, tau tangles are often better correlated with clinical outcomes than amyloid burden alone.
The intricate formation of these tangles provides insight into why memory and executive function deteriorate in Alzheimer’s patients. Tau protein in brain regions involved in cognitive processing becomes increasingly toxic, resulting in structural and functional disintegration. Understanding this mechanism is pivotal for developing therapies aimed at halting or reversing neurodegenerative changes. Therapies targeting tau phosphorylation, aggregation, and spreading are among the most promising areas of current Alzheimer’s research.
Amyloid Plaques and Tau Tangles: A Synergistic Pathology
Although amyloid plaques and tau tangles have distinct biochemical origins, their co-occurrence is particularly harmful to neural integrity. Emerging evidence suggests that amyloid beta and tau interact in ways that exacerbate each other’s toxic effects. The hypothesis that amyloid plaques and tau tangles form a synergistic pathology is gaining traction in scientific communities. According to this model, early amyloid deposition initiates a cascade of events that leads to tau pathology, including increased phosphorylation and misfolding.
This interplay is most evident in studies demonstrating that the presence of beta amyloid plaques accelerates the spread of tau tangles from one neuron to another. This progression correlates strongly with the onset of clinical symptoms, especially memory impairment and cognitive dysfunction. Thus, the combined pathology of amyloid plaques and neurofibrillary tangles may offer a more comprehensive explanation for the cognitive decline observed in Alzheimer’s patients than either pathology alone.
One intriguing line of inquiry explores how cerebral plaque—an umbrella term encompassing both amyloid and tau aggregates—can disrupt not only localized brain function but also long-range neural networks. Functional MRI studies have revealed altered connectivity in networks such as the default mode network, which is essential for introspective thought and memory. These disruptions reflect the widespread influence of plaques and tangles on both micro- and macro-level brain processes.

What Causes Amyloid Plaques and Tangles to Form?
Understanding what causes amyloid plaques and tau tangles to form remains a central challenge in Alzheimer’s research. Genetic factors, particularly mutations in the APP, PSEN1, and PSEN2 genes, have been identified in familial forms of Alzheimer’s and contribute to increased amyloid production or impaired clearance. In sporadic Alzheimer’s, the most common form, the apolipoprotein E (APOE) gene—especially the ε4 variant—is a well-established risk factor that influences amyloid deposition and tau pathology.
Environmental and lifestyle factors also appear to contribute to the formation of nerve plaque. These include chronic stress, poor sleep quality, sedentary behavior, and vascular conditions such as hypertension and diabetes. Oxidative stress and chronic inflammation can create a biological environment conducive to protein misfolding and aggregation. Researchers have also investigated the role of infections and microbiota changes in modulating amyloid and tau dynamics, adding further complexity to the picture.
Recent advances in imaging and cerebrospinal fluid biomarkers have allowed for earlier detection of amyloid beta and tau abnormalities, offering new insights into the timeline of disease progression. Interestingly, studies have shown that amyloid plaques in brain tissue can begin forming decades before clinical symptoms appear, highlighting the need for preventive strategies targeting early pathological changes. Likewise, efforts to understand how beta amyloid plaques and neurofibrillary tangles evolve over time are essential for developing stage-specific interventions.
The Role of Immune and Inflammatory Responses
Inflammation plays a substantial role in modulating how amyloid and tau exert their effects in Alzheimer’s disease. Microglia, the brain’s resident immune cells, are activated in response to the presence of amyloids in the brain and attempt to clear them. However, chronic activation can lead to sustained inflammation, which in turn damages neurons and exacerbates tau pathology. This inflammatory feedback loop creates a toxic environment where plaques and tangles continue to accumulate, intensifying disease progression.
Some researchers now view Alzheimer’s as a neuroinflammatory condition as much as a proteinopathy. The inflammatory milieu, characterized by cytokine release, oxidative damage, and blood-brain barrier dysfunction, appears to facilitate the misfolding of tau and the formation of amyloid plaques. Moreover, immune-related genes have been linked to Alzheimer’s susceptibility, emphasizing the interplay between genetic predisposition and immune dysfunction.
New treatments under investigation include anti-inflammatory agents and immunotherapies that target amyloid and tau directly. Monoclonal antibodies aimed at neutralizing beta amyloid plaques and tau tangles have shown mixed results, but they represent a critical avenue for reducing the toxic burden of cerebral plaque. The hope is that by mitigating inflammation, the brain’s natural clearance mechanisms can be reactivated, potentially slowing or halting disease progression.
Plaques and Tangles Contain Which of the Following: A Closer Look at Their Composition
The phrase “plaques and tangles contain which of the following” often appears in clinical and academic assessments to test knowledge of Alzheimer’s pathology. To clarify, amyloid plaques primarily consist of insoluble aggregates of amyloid beta peptides, particularly the Aβ-42 isoform. These peptides are sticky and prone to forming fibrils that clump together between neurons. The extracellular nature of these aggregates contributes to synaptic toxicity and inflammation.
Tau neurofibrillary tangles, by contrast, are composed of hyperphosphorylated tau protein. These intracellular inclusions disrupt the structural integrity of neurons and contribute to their eventual death. While they begin in localized regions of the brain, tau tangles spread in a prion-like manner, potentially by transferring misfolded tau from one cell to another. This spreading behavior underscores the importance of early intervention, as halting propagation may preserve brain function.
Importantly, both plaques and tangles are associated with secondary pathological changes, including the accumulation of other misfolded proteins, oxidative stress markers, and disrupted neuronal architecture. Understanding the molecular makeup of amyloid plaques and tau tangles allows for the design of targeted diagnostics and therapeutics. Research is ongoing to determine whether removing these pathological features can restore brain function or merely halt further damage.
The Clinical Implications of Amyloidosis in Alzheimer’s Disease
Amyloidosis in Alzheimer refers to the pathological accumulation of amyloid protein in brain tissue. This condition contributes not only to memory loss and confusion but also to broader disruptions in brain function. Clinical manifestations may include changes in personality, difficulty with language, impaired judgment, and loss of spatial awareness. As the disease progresses, these symptoms become increasingly severe and interfere with daily functioning.
Diagnosing amyloidosis Alzheimer traditionally relied on post-mortem brain analysis, but advances in neuroimaging and fluid biomarkers have made it possible to detect cerebral plaque in living patients. Positron emission tomography (PET) scans using amyloid tracers can identify amyloid plaques in brain regions associated with Alzheimer’s, while cerebrospinal fluid analysis can detect changes in amyloid beta and tau protein levels. These tools are now essential for confirming diagnoses and enrolling patients in clinical trials.
Importantly, amyloid burden alone does not guarantee the onset of Alzheimer’s symptoms. Many older adults show significant amyloid pathology without cognitive impairment, a phenomenon sometimes referred to as asymptomatic or preclinical Alzheimer’s. This observation underscores the need to understand the interplay between amyloid beta and tau, as well as additional factors such as resilience, cognitive reserve, and lifestyle.
Therapeutic Strategies Targeting Plaques and Tangles
Given the central role of amyloid and tau in Alzheimer’s disease, numerous therapeutic strategies have been designed to target these proteins. Some approaches focus on reducing the production of amyloid beta by inhibiting the enzymes involved in its formation. Others aim to promote clearance using immunotherapy or enhance degradation through molecular chaperones. Anti-amyloid monoclonal antibodies, such as aducanumab and lecanemab, have reached clinical trials and gained conditional approval, although their efficacy and safety continue to be debated.
Targeting tau is equally important. Researchers are exploring drugs that prevent tau phosphorylation, aggregation, or intercellular transfer. Small molecules, antisense oligonucleotides, and vaccines are among the modalities under investigation. Because tau pathology more closely correlates with disease severity, tau-targeted therapies may offer better outcomes, especially when used in combination with anti-amyloid treatments.
Ultimately, a multifaceted approach may be necessary. This could involve combining therapies that target amyloid plaques and tau tangles with interventions addressing neuroinflammation, oxidative stress, and metabolic dysfunction. Personalized medicine—tailoring treatment based on genetic, biomarker, and clinical profiles—is likely to play an increasingly important role in managing Alzheimer’s disease.
The Future of Alzheimer’s Research and Prevention
As science advances, there is growing emphasis on prevention and early intervention. Recognizing the presence of amyloid beta and tau decades before clinical symptoms arise opens the door to lifestyle modifications and medical interventions that could delay or prevent disease onset. Emerging data suggest that physical exercise, intellectual engagement, sleep hygiene, and cardiovascular health can influence the accumulation of amyloid plaques and neurofibrillary tangles.
Public health efforts now focus on raising awareness of modifiable risk factors and promoting brain-healthy habits across the lifespan. Simultaneously, researchers continue to explore novel imaging techniques, blood-based biomarkers, and artificial intelligence tools to improve early detection and prognosis. As our understanding of beta amyloid plaques and tau tangles deepens, so too does our ability to combat the devastating impact of Alzheimer’s disease.
While no cure currently exists, the tide is shifting. Multidisciplinary collaborations across neurology, immunology, molecular biology, and data science are accelerating progress. By targeting the root causes of plaque on the brain and unraveling the complex dynamics of tau in Alzheimer, future therapies may not only halt progression but one day prevent the disease altogether.
Frequently Asked Questions: Understanding Amyloid and Tau in Alzheimer’s Disease
1.What distinguishes amyloid plaques from tau tangles in terms of their impact on brain connectivity?
Amyloid plaques tend to disrupt neural communication across widespread cortical areas, leading to more diffuse cognitive effects early in disease progression. In contrast, tau tangles, particularly tau neurofibrillary tangles, impair localized neuronal transport, especially in memory-related structures like the hippocampus. Recent imaging studies show that while amyloid beta initiates broad synaptic dysfunction, tau tangles often correlate more strongly with functional disconnection in specific brain networks. When both plaques and tangles accumulate, they synergistically compromise long-range connectivity, accelerating cognitive decline. The overlapping presence of amyloid plaques and tau tangles explains why many individuals experience a rapid loss of executive function once both pathologies become widespread.
2.Are there protective genetic traits that reduce susceptibility to amyloid Alzheimer pathology?
While much research focuses on risk-enhancing genes like APOE ε4, emerging studies have identified protective alleles that appear to decrease the formation of beta amyloid plaques. For instance, certain rare mutations in the APP gene actually reduce the cleavage of amyloid precursor protein into amyloid beta, leading to fewer amyloid plaques in the brain. Similarly, variants in the KL gene (associated with klotho protein) have been linked to reduced accumulation of cerebral plaque and better cognitive resilience despite underlying pathology. These genetic findings offer promising avenues for future therapies that aim to mimic the protective mechanisms found in resistant populations. Understanding how these traits modulate amyloid beta and tau interactions may illuminate new preventive strategies for amyloidosis Alzheimer.
3.How does sleep quality affect the clearance of amyloid beta and tau from the brain?
Sleep is not merely restorative for mood and energy but plays a critical role in metabolic waste clearance, particularly of amyloids in the brain. During deep, slow-wave sleep, the brain’s glymphatic system becomes more active, helping remove amyloid beta and tau protein accumulations. Disrupted sleep patterns—especially reductions in REM and deep sleep—have been associated with increased beta amyloid plaques and tau neurofibrillary tangles. This connection has led researchers to explore sleep-focused interventions, such as cognitive behavioral therapy for insomnia (CBT-I), as a non-pharmaceutical way to reduce the risk of developing amyloid Alzheimer. Promoting regular, high-quality sleep may thus serve as a practical tool for slowing or preventing cerebral plaque buildup.
4.Can diet influence the development of amyloid plaques and neurofibrillary tangles?
Yes, dietary patterns can significantly influence the biological processes that promote or prevent amyloid plaques in brain tissue. Diets rich in antioxidants, omega-3 fatty acids, and polyphenols—such as the Mediterranean or MIND diets—have been shown to reduce oxidative stress and inflammation, two key drivers of both amyloid and tau pathology. Conversely, high consumption of processed sugars and saturated fats has been linked to increased nerve plaque and tau tangles through mechanisms involving insulin resistance and mitochondrial dysfunction. Certain flavonoids may even help prevent the aggregation of amyloid beta and tau proteins, offering a nutritional strategy for modulating these pathologies. Thus, food choices play a tangible role in determining what causes amyloid plaques to form or worsen over time.
5.How early can amyloid plaques and tau tangles be detected before symptoms appear?
Recent advances in imaging and fluid biomarkers now allow detection of both amyloid protein in brain tissue and phosphorylated tau in cerebrospinal fluid up to 20 years before clinical symptoms emerge. PET scans using radiotracers can reveal beta amyloid plaques and tau neurofibrillary tangles in asymptomatic individuals, often long before noticeable memory loss. Blood tests measuring specific forms of amyloid beta and tau are also becoming more precise and accessible, enabling earlier intervention. Detecting plaques and tangles this early opens the door to proactive treatment and lifestyle strategies, potentially delaying symptom onset. As research continues to refine these tools, early detection may soon become a standard part of preventive cognitive health care.
6.What role does chronic stress play in amyloid and tau accumulation?
Chronic psychological stress influences neurodegenerative pathways by increasing glucocorticoid levels, which can damage the hippocampus and impair protein regulation. Elevated cortisol levels have been linked to increased amyloid plaques and tau tangles in both animal models and human studies. Stress may also disrupt blood-brain barrier integrity, facilitating the infiltration of inflammatory mediators that promote amyloid beta and tau aggregation. Importantly, stress-reduction practices such as mindfulness, exercise, and social connection have shown promise in modulating these biological pathways. Addressing stress is not just a mental health concern—it is a crucial intervention for reducing the risk of amyloid plaques in the brain causes.
7.Is it possible to reverse amyloid and tau pathology once it has started?
While completely reversing established beta amyloid plaques and tau neurofibrillary tangles remains challenging, emerging therapies aim to slow or partially reverse their effects. Monoclonal antibodies targeting amyloid beta, such as lecanemab, have shown some success in reducing plaque on the brain in early-stage patients. Experimental treatments are also exploring tau aggregation inhibitors and antisense oligonucleotides that prevent abnormal tau formation. Lifestyle interventions, including aerobic exercise and cognitive training, have demonstrated modest but meaningful effects in reducing the progression of cerebral plaque. Although these strategies may not eradicate all amyloid and tau pathology, they offer hope for functional improvement and prolonged quality of life.
8.What distinguishes plaques and tangles in terms of their cellular location and spread?
Amyloid plaques typically accumulate outside neurons in the extracellular space, forming dense aggregates of beta amyloid peptides. Tau tangles, by contrast, form within neurons as twisted fibrils of tau protein that disrupt intracellular transport. This distinction influences how these pathologies propagate: amyloid plaques tend to appear diffusely and early, while tau tangles follow a more structured progression tied to symptom severity. Studies examining “plaques and tangles contain which of the following” often emphasize their unique compositions and locations to highlight diagnostic differences. Recognizing how amyloid plaques and tau tangles spread through different brain systems helps clinicians interpret imaging data and personalize interventions.
9.Are there cultural or socioeconomic factors that influence amyloid Alzheimer outcomes?
Yes, social determinants of health play a critical role in shaping outcomes in amyloidosis Alzheimer. Populations with limited access to healthcare, nutritious food, or educational resources may face greater risk due to both environmental and systemic barriers. Lower socioeconomic status has been associated with higher levels of beta amyloid plaques and tau pathology, potentially due to chronic stress, inadequate preventive care, or limited health literacy. Cultural stigmas surrounding dementia can also delay diagnosis and reduce access to early intervention. Addressing these disparities is essential not only for equity but also for reducing the burden of amyloid beta and tau-related cognitive decline across diverse communities.
10.How might future research alter our understanding of what causes plaque in brain tissue?
Future research is increasingly focused on the multifactorial origins of cerebral plaque, shifting from a single-cause model to a systems-based understanding. Investigations into how gut microbiota influence amyloid beta and tau metabolism are opening new frontiers in Alzheimer’s prevention. Additionally, machine learning tools are being used to predict who is most at risk for b amyloid plaques and neurofibrillary tangles based on genetic, lifestyle, and biomarker data. Researchers are also studying how environmental toxins and infections may contribute to what causes plaque in brain tissue by altering immune responses or promoting oxidative stress. These evolving insights promise to refine both our theoretical models and practical approaches to mitigating amyloid and tau pathology.
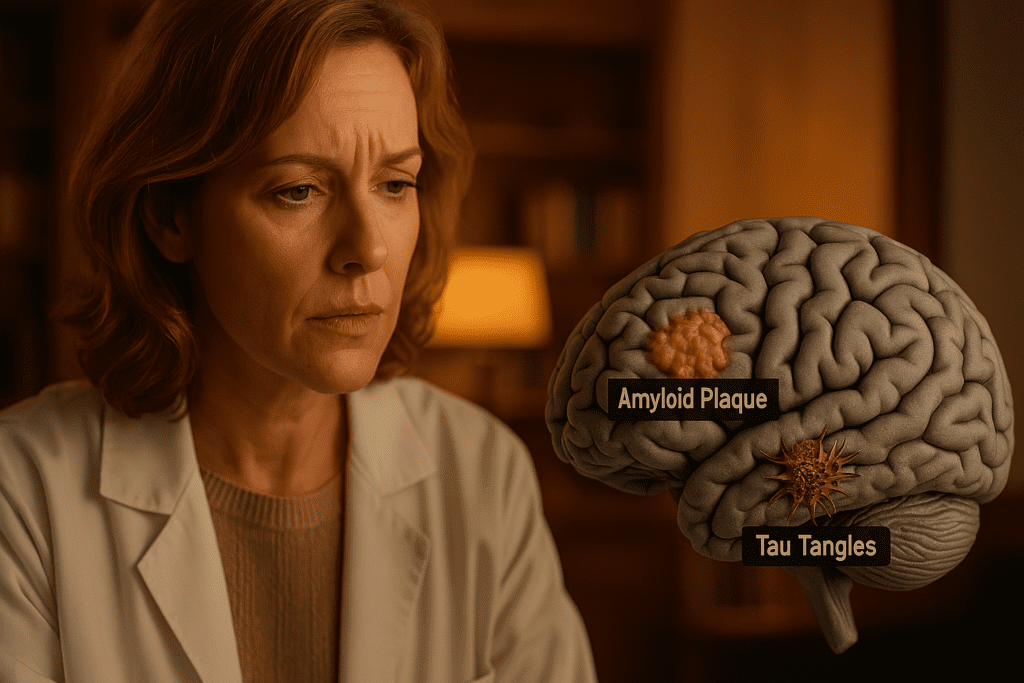
Conclusion: Unraveling the Dual Threat of Amyloid and Tau in Alzheimer’s Disease
Understanding the complex interplay of amyloid plaques and neurofibrillary tangles offers a window into the fundamental processes driving Alzheimer’s disease. These two proteinopathies—each devastating in its own right—become especially destructive when they intersect. From the initial deposition of amyloid beta to the insidious spread of tau tangles, the cumulative impact on brain function is profound. By illuminating how amyloid plaques in the brain cause dysfunction and how tau neurofibrillary tangles disrupt intracellular systems, researchers are paving the way toward more accurate diagnostics and effective treatments.
The synergy between beta amyloid plaques and tau tangles reflects a deeper biological narrative—one involving genetic susceptibility, environmental triggers, and age-related vulnerability. Interventions that disrupt this narrative, whether through medication, lifestyle changes, or early detection, offer new hope in managing amyloidosis Alzheimer. As our scientific understanding expands, so too does the potential for a future in which the devastating consequences of plaques and tangles are not an inevitability but a preventable outcome. With continued research and public awareness, the goal of transforming Alzheimer’s from a terminal condition to a manageable one is increasingly within reach.
brain protein aggregates, neurodegenerative disorders, memory loss prevention, synaptic dysfunction, cognitive decline research, dementia biomarkers, protein misfolding in the brain, Alzheimer’s early detection, neurological inflammation, hippocampal damage, brain health science, proteinopathy in aging, Alzheimer’s treatment development, cognitive resilience, neuroimaging in dementia, protein accumulation in neurons, aging and brain health, neurological disease risk factors, brain detox during sleep, lifestyle impact on brain aging
Further Reading:
The Intersection of Amyloid Beta and Tau at Synapses in Alzheimer’s Disease
Integrating amyloid and tau imaging with proteomics and genomics in Alzheimer’s disease
Disclaimer
The information contained in this article is provided for general informational purposes only and is not intended to serve as medical, legal, or professional advice. While Health11News strives to present accurate, up-to-date, and reliable content, no warranty or guarantee, expressed or implied, is made regarding the completeness, accuracy, or adequacy of the information provided. Readers are strongly advised to seek the guidance of a qualified healthcare provider or other relevant professionals before acting on any information contained in this article. Health11News, its authors, editors, and contributors expressly disclaim any liability for any damages, losses, or consequences arising directly or indirectly from the use, interpretation, or reliance on any information presented herein. The views and opinions expressed in this article are those of the author(s) and do not necessarily reflect the official policies or positions of Health11News.