Introduction: Why Spinal Cord Pathways Matter for Brain and Body Health
The human nervous system is a marvel of biological engineering, and at its core lies the spinal cord—a conduit not only for movement and sensation but also for a continuous exchange of information between the brain and the body. Understanding how spinal cord pathways work and function is critical for anyone interested in mental health, neurological function, or even physical well-being. These pathways are far more than anatomical structures; they are the essence of how we perceive pain, coordinate movement, maintain balance, and even regulate mood. In recent years, interest in spinal cord health has surged—not only due to its relevance in injury and disease but also because of its role in cognitive and emotional regulation. This article explores how these intricate networks work, diving into the importance of first, second, and third-order neurons, as well as the structural complexity illustrated in any standard spinal tracts diagram. This knowledge is vital for understanding how conditions like multiple sclerosis, chronic pain syndromes, or spinal cord injuries disrupt not just physical movement but also cognitive and emotional stability.
In the broader context of health and wellness, spinal cord pathways act as vital mediators of communication between the brain and peripheral systems. They do more than merely relay sensory or motor signals; they influence our capacity to respond to our environment, to recover from trauma, and to adapt to ongoing mental or physical stressors. As we unpack the functions of spinal nerve pathways and examine the role of individual neurons in both ascending and descending tracts, we will also explore how disruptions in these systems affect long-term brain health. Additionally, by examining how the number of neurons in a descending pathway is structured and functions, we shed light on broader implications for rehabilitation and neuroplasticity. Through this lens, spinal cord pathways become central to understanding not just physical disorders but also how neurobiology influences our everyday mental and emotional lives.
You may also like: Boost Brain Power Naturally: Evidence-Based Cognitive Training Activities and Memory Exercises That Support Long-Term Mental Health
Understanding the Architecture of Spinal Cord Pathways
The spinal cord, though only about 45 centimeters in length, contains one of the most complex networks of neuronal tracts in the human body. These spinal cord pathways are broadly categorized into ascending and descending tracts, each responsible for transmitting different types of information. Ascending tracts carry sensory data from the peripheral body to the brain, while descending tracts relay motor commands from the brain to the body. Each of these systems is highly specialized and utilizes a defined number of neurons, often categorized as first, second, and third order neurons, to relay information with extraordinary speed and precision.
When examining a spinal tract diagram, one can appreciate the elegant organization of these networks. For instance, the dorsal column-medial lemniscal pathway carries fine touch and proprioceptive information using a three-neuron chain, involving a first order neuron that enters the spinal cord, synapses with a second order neuron in the medulla, and finally communicates with a third order neuron in the thalamus that projects to the cerebral cortex. This organization is not arbitrary—it represents a highly evolved mechanism to ensure that critical information such as pain, temperature, or vibration is accurately processed and interpreted. Understanding this architectural arrangement is essential for clinicians, neuroscientists, and anyone interested in how bodily sensation relates to conscious experience.
The descending tracts follow a similar logic but in reverse. The number of neurons in a descending pathway is generally fewer, often involving an upper motor neuron originating in the cortex and a lower motor neuron that exits the spinal cord to innervate muscles. However, additional interneurons and modulatory cells may be involved, particularly in complex motor tasks. Appreciating the distinct anatomical and functional layers of these spinal cord pathways provides the foundation for interpreting both physiological function and pathological dysfunction, including how disruptions in signal transmission can lead to spasticity, paralysis, or chronic neuropathic pain.
The Role of First-Order Neurons in Sensory Integration
First order neurons play a critical role in initiating sensory input, forming the initial link in the chain of information that ascends through the spinal cord to the brain. Located in the dorsal root ganglia, these neurons have peripheral processes that reach out to the skin, muscles, and organs and central processes that project into the spinal cord. Their job is to transduce mechanical, chemical, or thermal stimuli into electrical signals that can be interpreted by the central nervous system. Without the accurate functioning of first order neurons, the body would lose its ability to interpret environmental signals, leading to a cascade of dysfunctions in perception, coordination, and protective reflexes.
In the context of spinal nerve pathways, these first-order neurons are gatekeepers of information. They determine not only what signals are transmitted but how fast and accurately these signals reach higher centers. This is particularly important in reflex arcs and in the modulation of pain, where rapid signal transmission can mean the difference between protection and injury. Moreover, the interaction between first order neurons and surrounding glial cells or immune mediators has been increasingly recognized as a factor in chronic pain conditions, illustrating that these neurons are not just passive relays but dynamic players in neural health.
The precision of the first order neuron function is so critical that even minor disruptions can have profound effects. For example, in diabetic neuropathy, the degeneration of these neurons leads to numbness, burning pain, and impaired proprioception, all of which can increase the risk of falls and other injuries. In neurodegenerative diseases, first-order neuron degradation can precede central nervous system involvement, acting as an early biomarker for more systemic neural decline. By understanding how these neurons function within spinal cord pathways, clinicians and researchers can better target therapies aimed at restoring normal sensation and preventing long-term neurological damage.
Second-Order Neurons: The Midpoint of Neural Processing
Second order neurons serve as the vital relay stations that integrate and refine sensory information before passing it upward to higher brain centers. Located primarily in the dorsal horn of the spinal cord or in the medulla oblongata, these neurons receive input from first-order neurons and transmit this data to the thalamus or cerebellum. Their role in the sensory pathway is critical for modulating the intensity, duration, and spatial quality of a stimulus. In practical terms, these neurons help the brain determine not only what is being felt but also how significant or urgent that sensation is.
One of the most fascinating aspects of second order neurons is their ability to engage in complex synaptic integration. These neurons receive converging signals from multiple first-order neurons, allowing for spatial summation, and can be influenced by descending modulatory pathways that alter their responsiveness. This interplay is central to phenomena like pain modulation, where the brain can either amplify or suppress sensory input depending on context. For example, the periaqueductal gray matter in the midbrain can activate descending pathways that inhibit second order neurons in the spinal cord, reducing the perception of pain—a process exploited by certain forms of cognitive behavioral therapy and mindfulness-based stress reduction.
Damage to second order neurons can produce distinctive clinical syndromes, such as Brown-Sequard syndrome, where a lesion on one side of the spinal cord leads to loss of pain and temperature sensation on the opposite side and motor weakness on the same side. This type of clinical presentation underscores the importance of understanding spinal cord pathways not only from a theoretical perspective but as essential knowledge for accurate diagnosis and treatment. Second order neurons thus represent a critical juncture in the nervous system—both a processing hub and a potential point of failure that can profoundly affect overall neurological health.
Third-Order Neurons: Bringing Sensory Experience to Consciousness
The journey of sensory data culminates in the activity of third order neurons, which project from the thalamus to the cerebral cortex, enabling the conscious perception of touch, temperature, pain, and proprioception. These neurons are the final relay in the classical sensory pathway and are essential for the cognitive interpretation of bodily sensations. Without the contribution of third order neurons, sensory information might still be transmitted and processed at lower levels, but it would not enter the realm of conscious awareness or subjective experience.
In terms of neuroanatomy, third-order neurons reside in the ventral posterior nuclei of the thalamus and send their axons through the internal capsule to reach specific areas of the somatosensory cortex. Here, the brain interprets incoming signals to generate meaningful perceptions—distinguishing between a light touch and a painful stimulus or identifying the location and texture of an object. This final step is not merely mechanical but is shaped by attention, expectation, and previous experience, highlighting the integrative role of the brain in creating a cohesive sense of self and environment.
Clinically, third order neurons are central to conditions involving central pain syndromes, such as thalamic pain syndrome, where damage to these neurons results in chronic, often debilitating pain that does not correspond to any ongoing tissue damage. This disconnect between stimulus and perception illustrates the complexity and importance of third order neuronal function within the spinal cord pathways. Therapies aimed at modulating thalamic activity—whether through pharmacological, electrical, or cognitive means—seek to restore balance in this final neural link. Thus, understanding third order neurons is indispensable for a complete view of how the spinal cord and brain integrate to produce coherent, adaptive behavior and perception.
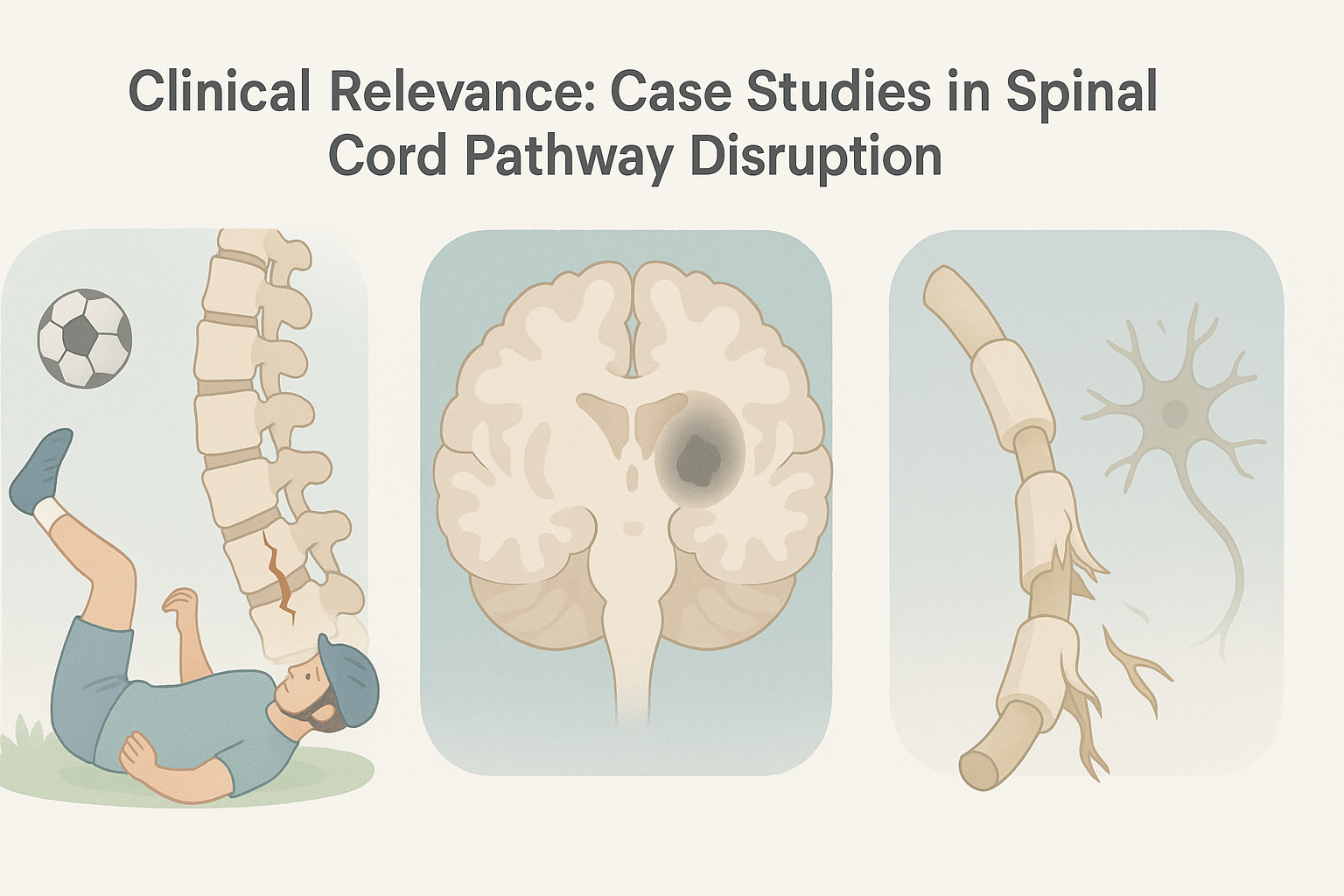
Clinical Relevance: Case Studies in Spinal Cord Pathway Disruption
Understanding spinal cord pathways is not just a theoretical exercise—it has real-world consequences for diagnosis, rehabilitation, and long-term neurological health. Consider the case of a young athlete who suffers a cervical spinal cord injury during a football game. The resulting damage to both ascending and descending tracts leads to partial paralysis and a loss of proprioception below the injury site. The analysis of a spinal tract diagram becomes more than an academic task; it becomes a roadmap for surgical planning, rehabilitation targets, and prognosis. Rehabilitation specialists use this understanding to customize physical therapy regimens, focusing on retraining intact spinal nerve pathways to compensate for lost functions through neuroplasticity.
In another case, a middle-aged woman presents with chronic, unexplained pain on one side of her body following a minor stroke. Neurological examination and imaging reveal damage to third order neurons in the thalamus, leading to central pain syndrome. Treatment options are limited, but understanding that the number of neurons in a descending pathway is relatively minimal helps focus therapeutic strategies on upstream modulation rather than peripheral interventions. Cognitive behavioral therapy, in combination with neuromodulatory medications, offers partial relief by engaging higher cortical centers that influence descending pain control pathways.
A third example involves a patient with multiple sclerosis (MS), an autoimmune condition that disrupts myelin in the central nervous system. In MS, demyelination affects not only first and second order neurons but can also interrupt the integrity of the spinal cord pathways themselves. As the disease progresses, it interferes with both motor and sensory tracts, causing unpredictable and fluctuating symptoms. Here again, an accurate interpretation of spinal tract diagrams and a solid understanding of neuron order can help neurologists differentiate between peripheral neuropathies and central demyelinating events. This distinction is critical for choosing appropriate disease-modifying therapies and for assessing the patient’s risk for future disability.
Each of these examples illustrates how the theoretical constructs of spinal cord anatomy directly inform clinical decision-making. Whether through mapping dysfunction on a spinal tract diagram, evaluating how many neurons are involved in a descending pathway, or targeting specific orders of neurons with therapy, the knowledge becomes actionable. This underscores the importance of integrating anatomical precision with clinical acumen in both research and practice. In doing so, health professionals are better equipped to address not only the physical manifestations of neural damage but also the cognitive and emotional toll such conditions impose.
Neuroplasticity and the Healing Potential of Spinal Cord Pathways
While spinal cord injury and disease often conjure images of irreversible loss, the concept of neuroplasticity provides a more hopeful outlook. Neuroplasticity refers to the nervous system’s remarkable ability to reorganize itself in response to injury, learning, or changes in the environment. Within the spinal cord, this adaptability is not only present but actively engaged during rehabilitation. Whether the damage involves a disruption in first, second, or third order neurons, or a complete severance of specific tracts spinal cord structures rely upon, the potential for rerouting information through alternate pathways remains an exciting frontier in both research and treatment.
One of the clearest demonstrations of neuroplasticity involves patients with partial spinal cord injuries who regain function through rigorous, targeted physical therapy. These individuals often engage the remaining intact spinal nerve pathways to perform tasks that were once thought impossible post-injury. Rehabilitation protocols frequently focus on high-repetition, task-specific training, which encourages the brain and spinal cord to recruit new neuronal circuits. In these cases, understanding how the number of neurons in a descending pathway is configured allows clinicians to design interventions that maximize the use of residual motor pathways while minimizing maladaptive compensatory behaviors.
Emerging technologies such as brain-computer interfaces (BCIs), electrical spinal stimulation, and regenerative stem cell therapies further highlight how neuroplasticity can be harnessed. For instance, epidural stimulation of the spinal cord has enabled some patients with complete injuries to voluntarily move paralyzed limbs, bypassing traditional descending tracts. This would not be possible without an intricate understanding of spinal tract diagrams and the role of interneuronal connectivity. In effect, these approaches simulate natural descending inputs, tricking the spinal cord into acting as if the brain is still issuing commands.
From a mental health perspective, the implications of neuroplasticity extend beyond movement. Individuals coping with long-term neurological trauma often face depression, anxiety, and cognitive deficits. Yet the adaptability of spinal cord pathways suggests a bidirectional relationship between neural recovery and psychological well-being. Interventions such as mindfulness, virtual reality therapy, and neuromodulation can support both structural and functional improvements, encouraging not only physical repair but also cognitive and emotional resilience. In sum, neuroplasticity redefines our understanding of spinal injuries and disorders—not as fixed endpoints but as evolving conditions with the potential for transformation and healing.
Aging and the Evolution of Spinal Cord Pathways Over Time
As we age, the human nervous system undergoes a range of structural and functional changes, many of which begin at the level of the spinal cord. These alterations are not merely incidental side effects of growing older; they significantly influence both cognitive and physical health. Age-related changes in spinal cord pathways can affect everything from balance and coordination to memory and attention. Specifically, the integrity of spinal nerve pathways begins to decline due to reduced myelination, neuronal loss, and a general slowing of synaptic transmission. These changes can be observed in the deterioration of both ascending and descending tracts that spinal cord systems rely on to maintain fluid communication between the brain and body.
One of the most notable consequences of aging on spinal cord function is diminished proprioception, or the body’s sense of its position in space. As first order neurons become less responsive or degenerate, the relay of positional data to second and third order neurons is impaired. This contributes to a higher risk of falls and mobility issues in older adults. At the same time, the number of neurons in a descending pathway may not change dramatically, but their efficiency and responsiveness can decline due to decreased neurotransmitter availability and receptor sensitivity. These age-related impairments collectively slow reflexes, weaken muscle strength, and reduce the capacity for fine motor control.
Another key factor is the cumulative burden of inflammation and oxidative stress. Chronic low-grade inflammation, often referred to as “inflammaging,” can disrupt normal signaling across spinal cord pathways. It affects glial cell activity and impairs the function of interneurons that support efficient neural transmission. These microscopic changes compound over time, eroding the overall performance of both motor and sensory circuits. For instance, disruptions in spinal nerve pathways have been linked to age-related gait disturbances and reduced coordination, which are not merely musculoskeletal in origin but are deeply rooted in neural degradation.
Despite these challenges, the aging spinal cord retains some capacity for plasticity and adaptation. Cognitive training, physical activity, and nutraceutical support—such as omega-3 fatty acids and B vitamins—have all been shown to enhance the health of neural pathways. Furthermore, targeted interventions such as resistance training and balance exercises can stimulate spinal tracts and promote the retention of function. An understanding of the spinal tracts diagram in older populations allows geriatric specialists to focus on preserving the most critical circuits and designing therapies that prioritize neurological efficiency.
Ultimately, the aging process reshapes the landscape of spinal cord pathways. Yet, with an informed approach that integrates knowledge of first, second, and third order neurons, clinicians and researchers can intervene more effectively. This proactive stance enables older adults not only to maintain physical autonomy but also to support long-term cognitive health by preserving the very communication networks that link body and brain.
Emotional Regulation and Mental Health: The Spinal Cord’s Hidden Role
The spinal cord is traditionally viewed as the anatomical foundation for reflexes and motor-sensory communication, but its involvement in emotional regulation and mental health has garnered increasing attention. The spinal cord is not simply a passive highway for signals—it actively participates in the modulation of mood, stress responses, and emotional behavior. By serving as the first processing point for sensory information, including pain and visceral sensations, spinal cord pathways can directly influence the limbic system, which governs emotional experiences. This connection is especially significant in understanding conditions like anxiety, depression, and post-traumatic stress disorder (PTSD).
When an individual encounters a stressor, sensory inputs—such as pain, threat signals, or internal discomfort—are processed initially by spinal nerve pathways. These inputs not only activate somatic reflexes but also initiate neurochemical cascades that feed into brain regions responsible for emotional responses. The hypothalamic-pituitary-adrenal (HPA) axis is one such downstream target, and it is partly governed by afferent signals that pass through ascending tracts and spinal cord structures. If these inputs are heightened due to inflammation, injury, or chronic pain, they can lead to hyperactivity in stress circuits, resulting in persistently elevated cortisol levels and long-term emotional dysregulation.
First and second-order neurons play a subtle but critical role in this loop. Chronic activation of these neurons in response to physical or emotional pain can reinforce maladaptive circuits through a process known as central sensitization. In PTSD, for instance, certain sensory stimuli may trigger disproportionate autonomic responses because the spinal cord has become conditioned to interpret benign input as threatening. This conditioning is not just psychological—it has a clear neurobiological basis rooted in the plasticity of spinal cord pathways. Understanding how the number of neurons in a descending pathway affects feedback regulation can offer insight into why top-down modulation of emotion—such as through mindfulness or cognitive therapy—can be effective.
Therapeutically, the relationship between spinal cord pathways and mental health has inspired new approaches in integrative medicine. Techniques like spinal cord stimulation (SCS), transcutaneous electrical nerve stimulation (TENS), and vagus nerve stimulation (VNS) have shown promise not only in alleviating physical symptoms but also in modulating emotional states. These interventions leverage the body’s natural circuitry to recalibrate both pain and affective processing. Furthermore, examining a spinal tract diagram can help clinicians identify key relay points that could be targeted for such neuromodulatory interventions.
Ultimately, emotional resilience is deeply intertwined with the functional integrity of the spinal cord. By appreciating how spinal tracts contribute to both bottom-up and top-down processes in mental health, researchers and clinicians can bridge the traditional gap between neurology and psychiatry. This holistic view promotes more comprehensive care—one that addresses both the neuroanatomical and psychological dimensions of human health.
Autonomic Function and Spinal Cord Pathways: Bridging Neural and Systemic Health
Beyond movement and sensation, spinal cord pathways also serve as critical conduits for autonomic regulation—controlling involuntary processes like heart rate, digestion, respiratory rhythm, and immune responses. These autonomic functions are orchestrated through both ascending sensory inputs and descending motor outputs that pass through the spinal cord en route to the autonomic ganglia and visceral organs. Disruptions to these spinal tracts can have cascading effects, not just on localized systems but on the entire body’s homeostasis.
The spinal cord is segmented into regions that govern sympathetic and parasympathetic outflows. The thoracolumbar segments house the sympathetic preganglionic neurons that mediate fight-or-flight responses, while the sacral regions manage parasympathetic functions tied to rest and digestion. The balance between these two systems is crucial for physiological stability. When examining a spinal tract diagram, one can trace how both types of autonomic signals originate in the brainstem or hypothalamus and are routed through specific descending pathways to their target regions. Understanding how these circuits interconnect is critical when treating disorders such as dysautonomia, irritable bowel syndrome, and even chronic fatigue.
First order neurons in the sensory autonomic system collect data from visceral organs—monitoring blood pressure, oxygen levels, and pH—and relay this information through second and third order neurons to higher brain centers that coordinate adaptive responses. In this way, the number of neurons in a descending pathway is not just a matter of anatomical categorization; it plays a direct role in determining how efficiently regulatory commands are transmitted. Impairments in these pathways—due to trauma, neurodegeneration, or chronic illness—can lead to imbalances such as orthostatic hypotension, bradycardia, or gastrointestinal dysfunction.
Moreover, the spinal nerve pathways interact closely with the immune system. Research has demonstrated that descending neural inputs can modulate immune cell activity, suggesting that spinal cord health indirectly influences inflammation and infection resilience. This neural-immune cross talk is particularly relevant in autoimmune disorders like multiple sclerosis and lupus, where spinal cord involvement may correlate with disease flare-ups and progression. Such insights are shifting how clinicians and researchers conceptualize systemic illness—moving toward a more integrated understanding that includes the health of spinal tracts as foundational to overall well-being.
Given this complexity, therapeutic approaches targeting autonomic dysregulation often include neurorehabilitation strategies that stimulate specific spinal tracts. Biofeedback, breathing exercises, and neuromodulation all leverage the plasticity of these pathways. Additionally, diagnostic imaging and functional assessments of spinal cord pathways are increasingly being used to evaluate autonomic function, especially in complex or idiopathic cases. In essence, the spinal cord is not merely a structural bridge between the brain and body—it is a regulatory hub that fine-tunes the entire human system.
Frequently Asked Questions: Advanced Insights into Spinal Cord Pathways and Neural Health
1. How do spinal cord pathways influence emotional memory and long-term psychological resilience?
Spinal cord pathways are intimately involved in the processing of emotionally salient stimuli, especially when those signals originate from visceral organs or pain receptors. The body’s response to chronic stress or trauma often begins with sensory data transmitted through the tracts spinal cord systems rely on to reach the brain. Emotional memories, particularly those linked to trauma, are encoded more strongly when there is heightened input through spinal nerve pathways. Over time, repeated activation of certain first second third order neurons reinforces these emotional circuits, potentially making individuals more vulnerable to anxiety or PTSD. Therapies that interrupt maladaptive signals via neuromodulation can help weaken these engrained pathways, allowing new neural associations to form and supporting greater psychological resilience.
2. Can spinal tracts adapt in cases of early developmental damage, such as in cerebral palsy or spina bifida?
Yes, neurodevelopmental disorders like cerebral palsy demonstrate the remarkable adaptability of spinal cord pathways. In these cases, the number of neurons in a descending pathway is often altered due to perinatal injury, yet the brain and spinal cord can still rewire through alternative tracts spinal cord structures retain. Children with these conditions may engage atypical spinal nerve pathways, compensating with heightened input from proprioceptive or vestibular systems. By targeting first second third order neurons through intensive therapy, some degree of voluntary control can often be regained or strengthened. Early intervention is key, as the younger nervous system exhibits heightened plasticity and can reroute functional circuits more readily than in adulthood.
3. How do changes in spinal tracts affect digestion, bladder control, or reproductive function?
Spinal cord pathways extend far beyond muscles and joints; they also regulate autonomic processes like digestion and urogenital control. Disruption of specific tracts spinal cord regions manage—especially those involving the sacral segments—can lead to incontinence, erectile dysfunction, or irritable bowel symptoms. This is because the number of neurons in a descending pathway responsible for autonomic control must remain intact for smooth regulation of these systems. Additionally, spinal nerve pathways carry feedback from the bladder, intestines, and reproductive organs to higher centers via first and second order neurons, allowing the brain to adjust sympathetic or parasympathetic output accordingly. Damage at any level—whether spinal or supraspinal—can produce lasting dysfunction unless addressed with targeted biofeedback or neuromodulatory therapies.
4. What role do spinal nerve pathways play in phantom limb syndrome after amputation?
Phantom limb pain offers a powerful demonstration of how spinal nerve pathways and third order neurons continue to function even after a limb is removed. The neural circuits within the tracts spinal cord systems use to convey somatosensory data remain active, and the brain still receives signals via first second third order neurons as though the limb were present. This miscommunication can result in vivid pain or sensation in the absent limb. Neuroplasticity allows for partial reorganization, but the number of neurons in a descending pathway may also need recalibration through visual-motor therapy, mirror therapy, or spinal stimulation. These interventions aim to “retrain” the brain and spinal cord to correctly interpret the absence of peripheral input.
5. Are spinal tracts involved in regulating circadian rhythms and sleep quality?
Emerging evidence suggests that certain spinal cord pathways contribute to circadian regulation, particularly via autonomic and sensory feedback loops. Input from the spinal nerve pathways influences melatonin production and hypothalamic function by relaying environmental cues like temperature and pain. Disruption in the tracts spinal cord systems rely on may increase sleep fragmentation or affect sleep architecture by altering signals that modulate parasympathetic tone. Additionally, irregularities in first second third order neurons—especially those transmitting visceral sensations—can exacerbate insomnia linked to chronic pain. While the number of neurons in a descending pathway may not directly regulate sleep, their influence on stress systems and hormonal balance indirectly impacts circadian function.
6. How might spinal tracts contribute to immune regulation and autoimmune disease progression?
Spinal cord pathways are increasingly recognized as mediators of neuroimmune interactions. For example, tracts spinal cord centers use to regulate autonomic output influence spleen and lymph node activity, which in turn affects immune response. When the number of neurons in a descending pathway is compromised by inflammation or demyelination, immune dysfunction may follow. Research on spinal nerve pathways suggests they also relay danger signals from peripheral tissues, priming immune cells through third order neurons involved in pain and interoception. In autoimmune conditions like multiple sclerosis, targeting these spinal tracts with neuromodulation or immunotherapy may help reduce neuroinflammation and prevent further neural degradation.
7. Can spinal tracts be strengthened or protected through exercise and lifestyle interventions?
Absolutely. Physical exercise enhances the efficiency of spinal cord pathways by promoting neurogenesis, vascular health, and synaptic strength. Functional movements like squats, yoga, or swimming activate multiple spinal nerve pathways simultaneously, reinforcing both motor and sensory tracts. This boosts the efficiency of first second third order neurons and may preserve the integrity of the number of neurons in a descending pathway that typically degrade with age or disuse. Resistance training, in particular, stimulates the proprioceptive tracts spinal cord regions use to support posture and reflexes. Diet and sleep also play a role by influencing inflammation and neurotransmitter balance, both of which affect how signals are processed along spinal tracts.
8. What are the implications of spinal tracts damage for mental focus and executive function?
Although not often discussed in cognitive neuroscience, damage to spinal cord pathways can subtly impair mental focus, decision-making, and executive control. Chronic pain or motor dysfunction—often resulting from disruptions in the tracts spinal cord networks contain—places cognitive load on the brain. This reduces bandwidth available for higher-order tasks like planning, memory, and attention. Moreover, when the number of neurons in a descending pathway is altered, compensatory neural networks can draw resources away from cortical centers involved in cognition. Third order neurons may also relay distorted sensory information, affecting one’s internal state and emotional tone, both of which influence mental performance.
9. How are spinal tracts being used in brain-computer interface (BCI) research and development?
Spinal tracts have become an exciting frontier in brain-computer interface technology. Recent innovations aim to stimulate or record from spinal nerve pathways to restore movement in individuals with paralysis. Understanding the configuration of first second third order neurons allows researchers to design BCIs that effectively bridge damaged sections of the spinal cord. These systems often bypass compromised regions by stimulating the number of neurons in a descending pathway below the injury, enabling voluntary movement. Additionally, spinal tracts diagrams inform electrode placement for optimal signal transmission, improving the performance and accuracy of these life-changing devices.
10. What future therapies are being explored for regenerating or repairing spinal cord pathways?
Regenerative medicine is rapidly evolving to address spinal cord injury and neurodegeneration. Techniques such as stem cell transplants, gene editing, and bioengineered scaffolds aim to rebuild damaged tracts spinal cord tissues rely on for motor and sensory function. These approaches seek to replenish or reroute the number of neurons in a descending pathway that may have been lost to trauma or disease. Advances in optogenetics and magnetic stimulation also offer ways to manipulate specific spinal nerve pathways in real time, reactivating dormant circuits. Researchers are even using spinal tracts diagrams to model individual patient anatomy for personalized treatment protocols that optimize the function of first second third order neurons.
Conclusion: Why Spinal Cord Pathways Hold the Key to Lifelong Neurological and Mental Health
The deeper we explore the structure and function of spinal cord pathways, the clearer it becomes that these neural highways form the backbone—literally and figuratively—of not just physical mobility but of mental clarity, emotional balance, and systemic health. From the earliest stages of sensory integration by first order neurons to the final, conscious perceptions shaped by third order neurons, every signal that travels through spinal tracts contributes to the internal equilibrium that allows us to think, feel, and move with purpose. This intricate system, visualized in every spinal tract diagram, is more than a biological structure—it is a dynamic, adaptable interface that connects our experiences to our physiology.
By understanding the number of neurons in a descending pathway and how those neurons communicate within the greater latticework of spinal nerve pathways, researchers and clinicians gain invaluable insight into how disruptions occur—and how recovery can be supported. Whether addressing a spinal injury, managing neurodegenerative decline, treating autonomic imbalances, or improving mental health, this knowledge empowers interventions that are both more targeted and more compassionate. It allows us to shift away from fragmented care and toward holistic strategies that consider the spinal cord not in isolation but as an integral part of whole-person health.
Moreover, this article highlights how spinal cord pathways adapt across the lifespan. While age and disease may alter the speed or fidelity of neural communication, neuroplasticity offers a counterbalance—an inherent capacity for renewal that can be encouraged through physical training, cognitive therapy, and emerging technologies. Likewise, the interplay between spinal pathways and the autonomic and emotional centers of the brain suggests exciting possibilities for the treatment of mood disorders, trauma, and psychosomatic illness. This reinforces the urgent need to include spinal cord health in broader discussions of mental and cognitive well-being.
Ultimately, the spinal cord serves as the bridge between mind and body, mediating not only mechanical functions but also emotional and cognitive states. It is a key to understanding how we respond to stress, how we heal from injury, and how we sustain mental resilience throughout life. As we continue to unravel the complexities of spinal tracts, spinal nerve pathways, and the layered roles of first, second, and third order neurons, we move closer to a future where health care is not only more effective but also more profoundly informed by the interconnectedness of the human nervous system.
Understanding spinal cord pathways, then, is not merely academic—it is a vital, actionable pursuit. It is the foundation upon which we can build a new paradigm of health, one that sees the spinal cord not just as a conduit for movement but as a living, learning, and responsive network essential to every aspect of human vitality.
Further Reading:
What are neural pathways and tracts?
Ascending and Descending Pathways in the Spinal Cord
An Historical Perspective: The Second Order Neuron in the Pain Pathway