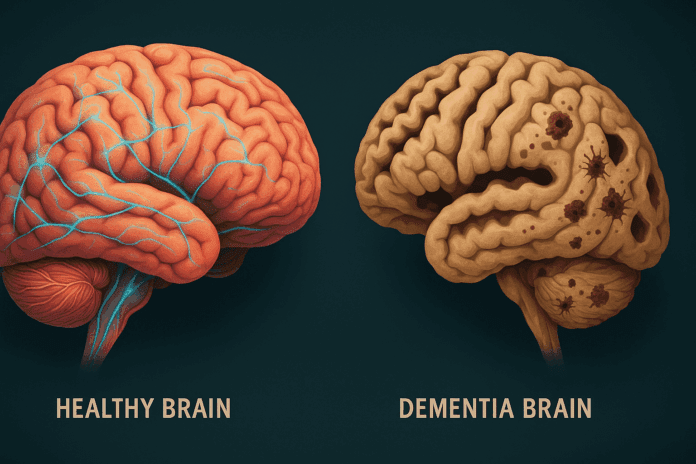
In an era where longevity is increasing, the distinction between maintaining cognitive health and succumbing to neurodegenerative decline has never been more critical. As scientists delve deeper into the architecture of the aging mind, the comparison between a Healthy Brain vs Dementia Brain offers profound insights. This article explores the fundamental differences between the healthy brain vs. the AD brain, often described in research as the contrast between the dementia brain vs. normal brain. By drawing on the latest neuroscience, we can better understand the biological, structural, and functional divergences that shape the cognitive trajectory of aging adults.
You may also like: How to Prevent Dementia and Alzheimer’s Disease Naturally: Expert-Backed Strategies to Reduce Your Risk Through Lifestyle and Diet
Understanding the Architecture of a Healthy Brain
A healthy brain is a marvel of biological engineering, maintaining its structural integrity and synaptic connectivity even into older age when well-supported by lifestyle and genetic factors. In a healthy adult brain, neuronal communication remains fluid, neural networks stay synchronized, and critical brain regions such as the hippocampus, prefrontal cortex, and temporal lobes continue to function effectively. These regions govern memory, executive function, emotional regulation, and spatial reasoning—all faculties essential for independent living.
Advanced neuroimaging studies reveal that a healthy brain retains a high level of plasticity, or the ability to form and reorganize synaptic connections. This plasticity is crucial for learning, adapting, and storing new information. In aging populations, neuroplasticity can be preserved or even enhanced through cognitive stimulation, physical activity, social engagement, and a diet rich in brain-supportive nutrients. At the biochemical level, healthy brains maintain a balanced environment of neurotransmitters like dopamine, serotonin, and acetylcholine, all of which play vital roles in mood, cognition, and attention.
From a morphological perspective, the cerebral cortex in a healthy brain remains relatively thick, and white matter tracts show minimal degradation. The blood-brain barrier stays intact, protecting neural tissue from potentially harmful agents in the bloodstream. Furthermore, there is minimal accumulation of beta-amyloid plaques or tau tangles—the pathological hallmarks of Alzheimer’s disease. Collectively, these characteristics define the resilient landscape of the healthy brain vs. AD brain.
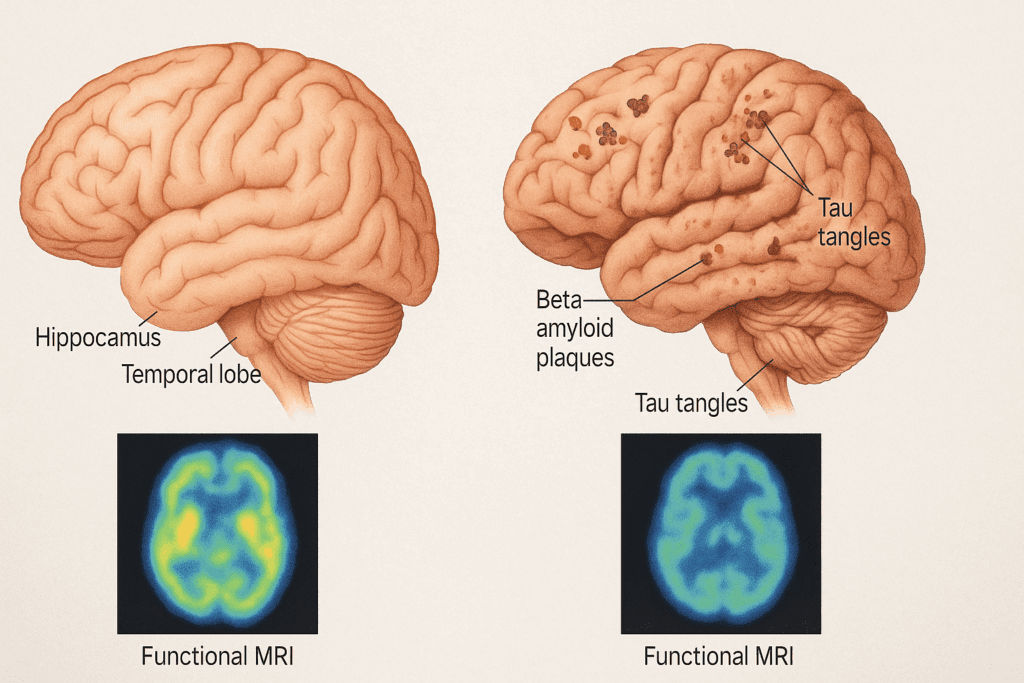
What Happens to the Brain in Dementia?
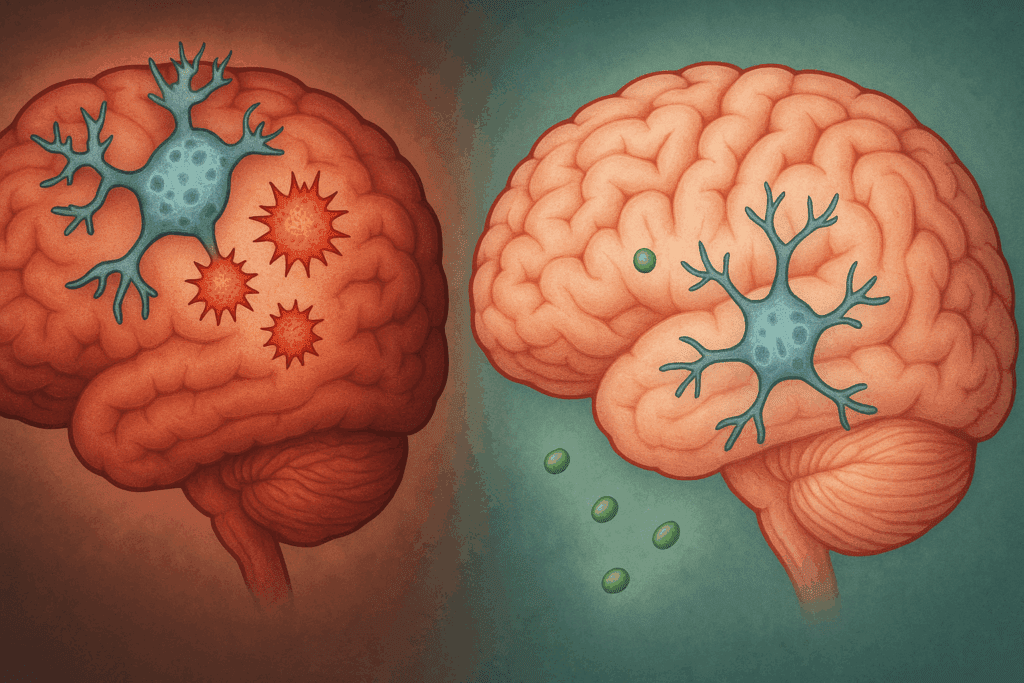
Dementia, particularly Alzheimer’s disease (AD), introduces a cascade of pathological changes that dramatically alter brain structure and function. The most striking feature when comparing the dementia brain vs. normal brain is the progressive atrophy, or shrinkage, of critical brain areas. The hippocampus, which is pivotal for memory consolidation, is among the first regions to suffer significant volume loss. As the disease advances, atrophy spreads to the temporal and parietal lobes, and eventually to the frontal cortex, undermining higher-order cognitive functions.
At the cellular level, neurons in the dementia brain degenerate and die, disrupting communication networks that underpin cognition. This loss is often precipitated by the abnormal accumulation of beta-amyloid plaques in the extracellular spaces and tau protein tangles within neurons. These misfolded proteins interfere with cellular transport systems, trigger inflammation, and ultimately lead to widespread synaptic failure. The healthy brain vs. AD brain shows clear divergence here: while the healthy brain is equipped to manage normal protein turnover, the dementia brain loses this capacity, leading to toxic buildup.
Functional MRI and PET scans in patients with Alzheimer’s also reveal marked reductions in brain metabolism, especially in the posterior cingulate cortex and temporoparietal junction. These areas, essential for memory and awareness of self, become hypoactive, correlating strongly with clinical symptoms. Such observations reinforce the notion that the dementia brain vs. normal brain represents not only structural degeneration but also a functional collapse of neural integration.
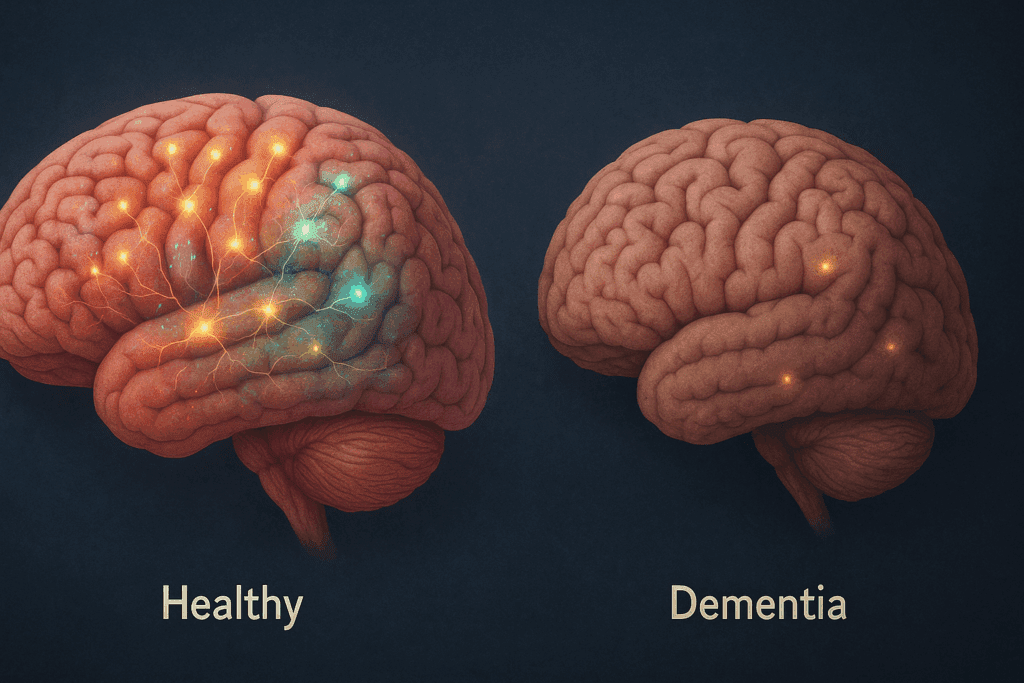
Cognitive Markers of Brain Health and Decline
Cognition serves as both the product and indicator of brain integrity. In a healthy individual, cognitive performance across domains such as memory, attention, language, and executive function remains relatively stable throughout adulthood, with only minor age-related declines. This maintenance of function reflects the robust neural connectivity and adaptability seen in a healthy brain.
In contrast, the cognitive trajectory of a person with dementia is marked by a persistent and accelerating decline. Early symptoms often manifest as difficulty remembering recent events, misplacing items, or struggling to find words. As the disease progresses, impairments extend to abstract thinking, planning, judgment, and eventually, basic daily activities. Importantly, the healthy brain vs. AD brain comparison helps clinicians differentiate between normal cognitive aging and pathological decline, aiding early diagnosis and intervention.
Neuropsychological testing supports these distinctions. Healthy adults typically score within the normal range on tests such as the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA), showing only mild reductions with age. In contrast, individuals with dementia exhibit substantial and progressive score reductions, particularly in memory recall, orientation, and complex task execution. The divergence in test results echoes the deeper structural and biochemical differences between the dementia brain vs. normal brain.
The Role of Inflammation and Oxidative Stress
Emerging research highlights the significant role of chronic inflammation and oxidative stress in distinguishing a healthy brain from one affected by dementia. In healthy aging, the brain’s immune system—primarily mediated by microglial cells—remains well-regulated, clearing out debris and maintaining neural homeostasis. However, in Alzheimer’s and related dementias, microglia become overactive, releasing pro-inflammatory cytokines that exacerbate neuronal damage.
This sustained inflammatory response contributes to a toxic environment in the brain, promoting the formation of beta-amyloid plaques and tau tangles. Additionally, oxidative stress caused by an imbalance between free radicals and antioxidant defenses leads to lipid peroxidation, DNA damage, and mitochondrial dysfunction. These processes collectively degrade neural integrity, sharply contrasting with the relatively stable biochemical milieu of the healthy brain.
Antioxidant-rich diets, regular physical exercise, and cognitive engagement have been shown to counteract inflammation and oxidative stress, reinforcing the importance of lifestyle in preserving cognitive health. By comparing the healthy brain vs. AD brain, researchers are now uncovering how targeted interventions may prevent or slow the progression of neurodegenerative diseases.
Connectivity and Communication in the Aging Brain
Another pivotal difference between a healthy brain and a dementia-affected brain lies in connectivity. In a healthy adult, neural networks remain coherent and functionally integrated. The default mode network (DMN), which is involved in self-referential thought and memory retrieval, remains highly active during rest and coordinated during cognitive tasks. Efficient connectivity between the DMN, salience network, and executive control network supports complex behaviors and decision-making.
However, in the dementia brain, especially in Alzheimer’s disease, these networks become disjointed. Functional disconnection among brain regions impairs information processing, leading to cognitive fragmentation. Studies using diffusion tensor imaging (DTI) have revealed that white matter tracts, such as the cingulum and corpus callosum, deteriorate significantly in dementia, disrupting interregional communication.
This contrast in connectivity underscores the neurological basis for cognitive decline. While the healthy brain continues to integrate sensory, motor, and executive signals efficiently, the dementia brain loses this orchestration, resulting in confusion, memory loss, and behavioral changes. The comparison of the dementia brain vs. normal brain in terms of network function has become a crucial focus of neuroscientific research aimed at early detection and therapeutic innovation.
Genetics, Risk Factors, and Preventive Strategies
Understanding the genetic and environmental risk factors that influence the transition from a healthy brain to a dementia-affected brain offers critical insight into prevention and management. The presence of the APOE4 allele is one of the most well-documented genetic risk factors for Alzheimer’s disease. Individuals with this allele have a significantly higher likelihood of developing dementia, although not all carriers will be affected. This highlights the interplay between genetic predisposition and lifestyle choices.
Modifiable risk factors such as hypertension, diabetes, obesity, and smoking have also been implicated in cognitive decline. These conditions contribute to vascular damage and reduced cerebral perfusion, further exacerbating neuronal vulnerability. In contrast, protective factors such as regular aerobic exercise, adherence to the Mediterranean or MIND diets, lifelong learning, and robust social networks have been associated with sustained cognitive function.
The emerging field of epigenetics suggests that gene expression can be modulated by environmental influences, offering hope for delaying or preventing dementia even in genetically at-risk individuals. By studying the healthy brain vs. AD brain through the lens of risk and resilience, researchers aim to develop personalized strategies that preserve mental acuity and delay disease onset.
Biomarkers and Advances in Early Detection
One of the most transformative developments in differentiating the dementia brain vs. normal brain has been the identification of biomarkers. Cerebrospinal fluid (CSF) analysis and blood-based biomarkers are increasingly used to detect beta-amyloid and phosphorylated tau levels long before symptoms appear. These indicators provide a window into the preclinical stages of Alzheimer’s, enabling earlier diagnosis and intervention.
Neuroimaging has also evolved dramatically. PET scans using amyloid- or tau-specific tracers allow for visualization of pathological burden in vivo. Magnetic resonance imaging (MRI) can detect early signs of cortical thinning, ventricular enlargement, and hippocampal atrophy. Such tools are revolutionizing clinical practice by enabling clinicians to track disease progression and evaluate the efficacy of therapeutic interventions.
The comparison of the healthy brain vs. AD brain through biomarker analysis provides a clearer roadmap for staging, prognosis, and treatment planning. As technology continues to advance, these methods hold the promise of transforming dementia care from reactive to preventive.
Toward a Neuroscientific Model of Healthy Brain Aging
While much attention has been given to understanding what goes wrong in dementia, equal emphasis must be placed on what goes right in healthy aging. Neuroscientific studies of super-agers—individuals in their 80s or older with cognitive abilities comparable to much younger people—offer valuable clues. These individuals often have larger-than-average cortical thickness in brain regions associated with attention and memory.
Additionally, super-agers exhibit lower levels of brain inflammation and greater resistance to age-related atrophy. Their lifestyles often include regular physical activity, intellectual engagement, meaningful relationships, and a positive outlook on life. These protective factors align closely with what distinguishes the healthy brain vs. AD brain, providing a practical framework for public health initiatives.
By embracing a neuroscientific model that focuses not only on disease pathology but also on the biological substrates of resilience, society can shift toward more holistic, preventative approaches to brain health. This model encourages the cultivation of lifelong habits that support neural integrity, from childhood education to elder wellness programs.
Frequently Asked Questions: Healthy Brain vs. Dementia Brain
1. What role does sleep play in distinguishing a healthy brain from one affected by dementia?
Sleep is emerging as a vital differentiator in the conversation about healthy brain vs. AD brain. A healthy brain relies on deep sleep to clear metabolic waste products, particularly beta-amyloid proteins that can accumulate into plaques. In contrast, the dementia brain vs. normal brain comparison reveals that individuals with Alzheimer’s disease often experience disrupted sleep architecture, with reduced slow-wave and REM sleep. This sleep disturbance can accelerate cognitive decline by impairing the glymphatic system’s waste-clearing functions. Prioritizing consistent, high-quality sleep may help slow progression toward neurodegeneration by mimicking the restorative cycles seen in a healthy brain.
2. How does diet influence long-term differences between a healthy brain and one developing Alzheimer’s?
While the article touches on diet, there is growing evidence that specific nutrients can create a metabolic environment favorable to the healthy brain vs. AD brain divide. Omega-3 fatty acids, flavonoids, and polyphenols have neuroprotective properties that enhance synaptic plasticity and reduce oxidative stress. In contrast, high-sugar, ultra-processed diets correlate with insulin resistance and inflammation—both of which are more common in the dementia brain vs. normal brain. Nutritional interventions like the MIND or Mediterranean diet not only lower Alzheimer’s risk but may also help preserve gray matter density. Emerging clinical trials are even examining ketogenic protocols for early cognitive impairment, suggesting future dietary strategies could target brain metabolism directly.
3. Are emotional resilience and mental health linked to brain aging outcomes?
Yes, the emotional landscape of a person plays a powerful role in distinguishing a healthy brain from one trending toward dementia. Chronic stress and untreated depression elevate cortisol levels, which have been shown to damage the hippocampus—a region disproportionately affected in the dementia brain vs. normal brain. On the other hand, emotional resilience, mindfulness, and access to meaningful social support can bolster neural connectivity and lower inflammatory markers. These psychological assets appear to create a protective buffer that sustains cognitive function even when biological risk factors are present. In this sense, emotional wellness becomes a pivotal element in the healthy brain vs. AD brain equation.
4. What are the long-term consequences of delayed diagnosis in dementia cases?
Delayed diagnosis blurs the critical window where early interventions can shift the trajectory between a healthy brain vs. AD brain outcome. The dementia brain vs. normal brain may look superficially similar in early stages, but subtle behavioral changes like apathy, social withdrawal, or mild executive dysfunction often go unnoticed. When diagnosis is delayed, patients miss out on disease-modifying therapies, clinical trials, and lifestyle interventions that are most effective before extensive neural loss occurs. Furthermore, delayed awareness can limit legal and financial planning, caregiver preparation, and patient autonomy. Timely recognition of symptoms allows for structured care strategies that enhance quality of life.
5. Can digital cognitive tools help differentiate between healthy and dementia-affected brains?
Digital platforms are becoming sophisticated enough to help clinicians distinguish between a healthy brain vs. AD brain, especially in early-stage assessments. Apps and software using AI-driven neurocognitive tasks can track micro-level changes in memory, processing speed, and decision-making over time. These tools can reveal deviations that might go undetected in standard office evaluations, especially when comparing the dementia brain vs. normal brain under real-world conditions. Some programs now integrate speech pattern analysis and eye-tracking, adding another layer of diagnostic insight. As technology becomes more personalized, digital cognition tracking may emerge as a front-line screening tool for high-risk populations.
6. What social and environmental factors influence brain aging beyond genetics?
Beyond the inherited risks often associated with Alzheimer’s, social determinants exert a profound impact on the healthy brain vs. AD brain distinction. Loneliness, air pollution, educational attainment, and neighborhood safety have all been linked to cognitive outcomes in aging. Individuals exposed to chronic environmental toxins or living in underserved areas may experience accelerated neural aging, leading to patterns consistent with the dementia brain vs. normal brain differences. On the flip side, cognitive reserve built through lifelong learning, creative hobbies, and bilingualism offers resilience against pathology. Addressing systemic inequities is therefore essential to mitigating dementia risk across communities.
7. Do healthy brains show any age-related decline, and how does it differ from dementia?
Yes, even the healthiest brains experience some age-associated changes, such as slower recall or minor lapses in multitasking. However, these changes remain within functional bounds and do not disrupt daily living, distinguishing them from the more pronounced impairments observed in the dementia brain vs. normal brain. Importantly, in a healthy brain vs. AD brain comparison, one can observe that the structural and metabolic integrity is largely preserved despite modest functional shifts. This underscores the concept of “normal aging” as distinct from pathological decline. Understanding this difference can help reduce unnecessary fear while promoting proactive health behaviors.
8. Are there cultural differences in how dementia is perceived or addressed?
Cultural perspectives play a significant role in shaping responses to the dementia brain vs. normal brain spectrum. In some cultures, cognitive decline is seen as a normal part of aging, which can delay recognition and treatment. Others may stigmatize dementia, discouraging families from seeking help. These differences affect how communities respond to early signs distinguishing a healthy brain vs. AD brain. Culturally competent care models, which respect beliefs while delivering science-based education, have proven effective in improving outcomes. Incorporating traditional wellness practices with modern interventions may also help bridge treatment gaps in diverse populations.
9. Can brain-training games or neurofeedback genuinely improve cognitive outcomes?
Brain-training tools and neurofeedback therapies have generated both enthusiasm and skepticism in discussions around the healthy brain vs. AD brain dynamic. While commercial products vary in quality, certain evidence-based programs demonstrate benefits in specific cognitive domains like working memory and reaction time. However, the dementia brain vs. normal brain does not simply differ in isolated skills—it diverges in structural integrity and neurochemical balance. Thus, brain training may be more effective as a component of a broader lifestyle intervention than as a standalone fix. Combined with exercise, nutrition, and social engagement, cognitive stimulation may help preserve function in at-risk individuals.
10. What does the future hold for distinguishing and treating different brain aging patterns?
Neuroscience is moving toward a more nuanced understanding of the healthy brain vs. AD brain continuum. Rather than treating dementia as a singular outcome, researchers now explore the dementia brain vs. normal brain as part of a diverse spectrum influenced by vascular, metabolic, and immune factors. Future diagnostics may combine blood biomarkers, genetic profiles, and wearable tech to create individualized brain health maps. Precision medicine approaches could tailor treatments based on each person’s risk profile, lifestyle, and disease stage. This vision promises a future where dementia is not only detected earlier but addressed with more targeted and effective strategies than ever before.
Conclusion: The Neuroscience Behind Brain Aging and Why It Matters
The comparison of the dementia brain vs. normal brain is more than an academic exercise—it is a powerful tool for understanding the trajectory of human cognition. By exploring the structural, functional, and biochemical differences that define the healthy brain vs. AD brain, we gain critical insights into how to preserve mental clarity, independence, and quality of life as we age.
Neuroscience has revealed that cognitive decline is not an inevitable consequence of aging but rather the result of complex interactions between genetics, lifestyle, and environmental exposure. While Alzheimer’s disease and other dementias remain formidable challenges, emerging evidence suggests that early intervention, targeted prevention, and personalized medicine can significantly alter outcomes.
Recognizing the early signs of divergence between a dementia brain and a healthy one allows for timely support, therapeutic strategies, and informed decision-making. It empowers individuals, families, and communities to prioritize cognitive well-being with the same urgency and dedication given to physical health. As our understanding deepens, so too does our ability to reshape the narrative of aging—transforming it from one of inevitable decline to one of sustained vitality and purpose.
In this light, the study of healthy brain vs. AD brain is not simply a scientific endeavor but a human one. It calls us to reimagine the aging process with compassion, evidence-based optimism, and a renewed commitment to brain wellness at every stage of life.
brain health strategies, cognitive aging research, signs of memory decline, neurodegenerative disease prevention, brain connectivity loss, early Alzheimer indicators, memory support techniques, synaptic health, hippocampal shrinkage, executive dysfunction, lifestyle for cognitive resilience, brain metabolism changes, personalized brain care, neuroinflammation and aging, oxidative brain stress, neuroplasticity in aging, cognitive reserve factors, digital brain health tools, biomarkers for brain health, mental wellness and cognition
Was this article helpful? Don’t let it stop with you. Share it right now with someone who needs to see it—whether it’s a friend, a colleague, or your whole network. And if staying ahead on this topic matters to you, subscribe to this publication for the most up-to-date information. You’ll get the latest insights delivered straight to you—no searching, no missing out.
Further Reading:
How the Aging Brain Affects Thinking