The human digestive system is a marvel of complexity, an elegant choreography of organs, enzymes, and biochemical signals working in harmony to convert food into the nutrients that sustain life. Yet beneath this apparent autonomy lies an intricate microbial world that is not only essential but foundational to digestive function. This world is built on symbiotic relationships—mutually beneficial partnerships between humans and the trillions of microbes that reside within our gut. As we explore how symbiotic relationships make digestion possible, it becomes clear that gut bacteria are not passive passengers; they are active, dynamic agents in human health. Their role is central to the mutualism relationship that defines the human microbiome, and understanding this interdependence is crucial for optimizing both physical and mental well-being.
You may also like: How Gut Health Affects Mental Health: Exploring the Gut-Brain Connection Behind Anxiety, Mood, and Depression
Understanding Symbiosis: The Foundation of Gut Ecology
In biological terms, symbiosis refers to any interaction between two different organisms living in close physical association. These interactions can be parasitic, commensal, or mutualistic. In the context of digestion, the relationship between gut bacteria and the human host is predominantly mutualistic. This means both parties benefit: humans provide a nutrient-rich environment for microbes to thrive, and in return, microbes perform essential tasks that the human body cannot do alone. It is precisely these mutualistic interactions that make digestion more than just a mechanical or chemical process. When asking what symbiotic relationships do you think make digestion possible, the clearest answer lies in the roles gut microbes play in breaking down otherwise indigestible fibers, synthesizing key vitamins, and modulating immune and metabolic functions.
The Gut Microbiome: A Living Digestive Organ
The gut microbiome is often described as an organ in its own right—dynamic, metabolically active, and indispensable. Comprising over 100 trillion microorganisms, primarily bacteria but also including fungi, viruses, and archaea, the gut microbiome varies greatly among individuals. However, certain core functions remain consistent. For example, many species of Bacteroides and Firmicutes produce enzymes that degrade complex polysaccharides into short-chain fatty acids (SCFAs), which serve as a vital energy source for colon cells. This enzymatic activity illustrates precisely how symbiotic relationships make digestion possible, especially in parts of the gastrointestinal tract that lack intrinsic enzymatic capability. Without microbial fermentation, humans would extract significantly fewer nutrients from their diet, particularly from plant-based sources.
Mutualism in Action: How Gut Bacteria Support Digestion
Mutualism in the gut is not merely theoretical; it is observable at every stage of digestion. From the moment food enters the gastrointestinal tract, bacteria begin to assist with decomposition and transformation. In the small intestine, microbial metabolites influence motility and nutrient absorption. In the large intestine, fermentation of dietary fibers produces SCFAs such as acetate, propionate, and butyrate. These molecules not only provide energy to host cells but also play roles in regulating inflammation, gut integrity, and even appetite. Understanding how gut bacteria affect humans in the mutualism relationship allows researchers and clinicians to appreciate the depth of these effects. It is not just a matter of aiding digestion—these bacteria actively shape metabolic pathways, hormonal regulation, and even neurological signaling.
Nutrient Synthesis: Beyond Digestion to Biosynthesis
One of the most striking ways in which gut microbes contribute to human health is through the synthesis of essential nutrients. While the human genome encodes for enzymes to digest proteins, fats, and carbohydrates, it lacks the machinery to produce certain vitamins—specifically, B vitamins such as B12, B6, folate, and biotin, as well as vitamin K. Enter the gut microbiota. Bacteria such as Lactobacillus and Bifidobacterium can synthesize these vitamins, which are then absorbed by the host. This biosynthetic capacity underscores how symbiotic relationships make digestion possible not only by assisting in the breakdown of food but also by enhancing the nutritional profile of what we consume. It also sheds light on why disruptions to the microbiome—such as those caused by antibiotics or dietary imbalances—can lead to nutrient deficiencies and systemic health issues.
Gut Bacteria and Immune Regulation
The relationship between gut microbes and the immune system is a paradigm of biological symbiosis. Approximately 70% of the immune system is located in the gastrointestinal tract, where it constantly interacts with gut flora. Far from being a defensive barrier alone, the immune system is trained and modulated by microbial exposure. Certain commensal bacteria encourage the development of regulatory T cells, which help maintain immune tolerance and prevent inflammatory diseases. In this context, understanding what symbiotic relationships do you think make digestion possible extends beyond nutrient processing to immune education. Without the microbial stimulation provided by a diverse gut microbiome, immune function can become either hyperactive—as in autoimmune diseases—or hypoactive, as seen in frequent infections.
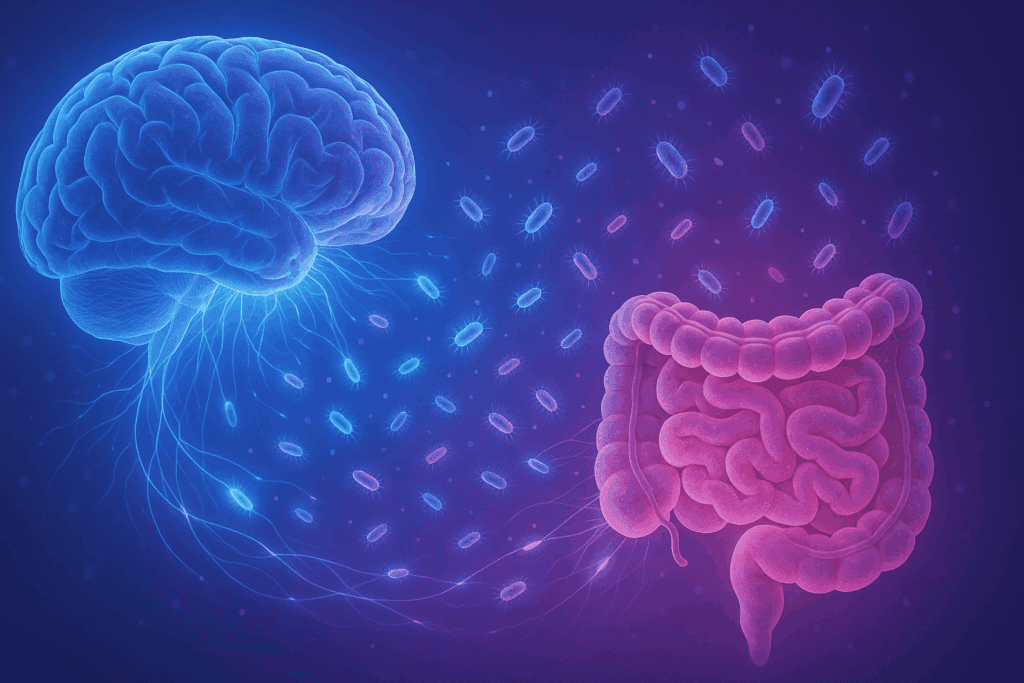
Gut-Brain Axis: Mental Health Implications of Digestive Symbiosis
Emerging research into the gut-brain axis reveals profound connections between digestion and mental health. The microbiota-gut-brain axis is a bidirectional communication network linking the central nervous system with the enteric nervous system of the gut. Microbes produce neuroactive compounds such as serotonin, dopamine, and gamma-aminobutyric acid (GABA), which influence mood, cognition, and behavior. When evaluating how gut bacteria affect humans in the mutualism relationship, one cannot overlook their impact on neuropsychological health. Disruptions in gut microbial composition have been linked to conditions such as depression, anxiety, autism spectrum disorders, and even neurodegenerative diseases. This underscores the necessity of maintaining microbial balance, not only for digestive integrity but also for cognitive resilience and emotional stability.
Dysbiosis: When Symbiosis Breaks Down
While symbiotic relationships are typically beneficial, they are also delicate and can be disrupted by various factors. Dysbiosis—a term for microbial imbalance—can result from antibiotics, poor diet, stress, environmental toxins, and infections. When harmful bacteria outcompete beneficial strains, the consequences ripple throughout the body. Symptoms may include bloating, diarrhea, constipation, and fatigue, but the effects can be more systemic as well, contributing to obesity, metabolic syndrome, and inflammatory bowel diseases like Crohn’s and ulcerative colitis. This fragility highlights the importance of asking what symbiotic relationships do you think make digestion possible, particularly when they begin to fail. The answer lies in a resilient, diverse microbiome, supported by diet, lifestyle, and—in some cases—probiotic or prebiotic interventions.
Dietary Fiber and Prebiotics: Feeding Our Microbial Partners
The health of the microbiome is closely tied to what we eat. Dietary fibers, especially prebiotics like inulin and fructooligosaccharides, serve as fuel for beneficial bacteria. These non-digestible carbohydrates reach the colon intact, where they are fermented by gut microbes into SCFAs and other metabolites. The regular consumption of fiber-rich foods supports microbial diversity, enhances barrier function in the gut lining, and reduces systemic inflammation. Here, the question of how gut bacteria affect humans in the mutualism relationship becomes particularly relevant. The food we consume determines the population and function of our microbial partners, directly influencing digestive efficiency, immune robustness, and mental clarity.

The Role of Probiotics and Fermented Foods
In recent years, there has been growing interest in probiotics—live microorganisms that confer health benefits when consumed in adequate amounts. Fermented foods such as yogurt, kefir, sauerkraut, kimchi, and miso are natural sources of probiotics, and their inclusion in the diet can help restore and maintain microbial balance. Clinical studies suggest that certain probiotic strains can alleviate symptoms of irritable bowel syndrome, reduce antibiotic-associated diarrhea, and even improve mood disorders. By enhancing mutualistic interactions within the gut, probiotics illustrate how symbiotic relationships make digestion possible even when native microbiota are compromised. Their therapeutic potential is vast, but it must be approached with scientific rigor and personalized application.
Infant Microbiota: The Early Stages of Symbiosis
The establishment of the gut microbiome begins at birth and continues to evolve throughout early childhood. Factors such as mode of delivery, breastfeeding, antibiotic exposure, and early diet play crucial roles in shaping microbial composition. Vaginally delivered infants tend to acquire beneficial bacteria from the mother’s birth canal, while those born via cesarean section may have delayed microbial colonization. Breast milk contains both probiotics and prebiotics, fostering a nurturing environment for early symbiosis. Understanding what symbiotic relationships do you think make digestion possible during infancy can provide insights into long-term health trajectories. Early disruptions to this delicate process may predispose individuals to allergies, autoimmune disorders, and even metabolic diseases later in life.
Environmental and Lifestyle Influences on the Microbiome
Beyond diet, a variety of environmental and lifestyle factors influence microbial health. Exposure to nature, physical activity, stress levels, and sleep quality all modulate the composition and function of the microbiome. Chronic stress, for instance, can alter gut permeability and microbial diversity, potentially leading to inflammation and digestive discomfort. Sleep deprivation has similarly been linked to microbiome disturbances. Thus, optimizing lifestyle habits can reinforce the mutualism between humans and their gut bacteria. Understanding how gut bacteria affect humans in the mutualism relationship requires a holistic approach that considers behavioral and psychosocial dimensions alongside nutritional and medical ones.
Fecal Microbiota Transplants: Restoring Symbiotic Balance
Fecal microbiota transplantation (FMT) has emerged as a powerful clinical intervention for restoring microbial balance, particularly in patients with recurrent Clostridioides difficile infections. By transplanting stool from a healthy donor into the gastrointestinal tract of a patient, clinicians can reestablish a functional microbiome and eliminate pathogenic bacteria. Although still an evolving field, FMT demonstrates how symbiotic relationships make digestion possible even after severe disruption. Research is ongoing to explore its potential in treating conditions like inflammatory bowel disease, obesity, and neuropsychiatric disorders. As our understanding of the gut microbiome deepens, so too does the range of possibilities for therapeutic manipulation.
Personalized Nutrition and the Future of Digestive Symbiosis
The future of digestive health lies in personalization. Advances in metagenomics and metabolomics now allow for individualized analysis of microbial composition and activity. Personalized nutrition, based on an individual’s unique microbiome, can optimize digestive outcomes and overall health. This shift from generic dietary advice to tailored interventions underscores the importance of knowing what symbiotic relationships do you think make digestion possible in your own body. As research continues to uncover the nuances of microbial function, the era of precision gut health promises targeted therapies that align with one’s genetic, microbial, and lifestyle profile.
Maintaining Microbial Harmony for Lifelong Wellness
To sustain the symbiotic relationships that underpin digestion, long-term strategies are essential. A diverse, plant-rich diet, regular physical activity, stress management, and adequate sleep form the foundation of microbial harmony. Avoiding unnecessary antibiotics, incorporating fermented foods, and using targeted probiotic supplements when needed can also support microbial resilience. Understanding how gut bacteria affect humans in the mutualism relationship helps us prioritize gut health as a cornerstone of holistic wellness. It is a continuous, dynamic interaction—one that evolves over the lifespan and requires conscious care.
Frequently Asked Questions (FAQ): How Symbiotic Relationships Make Digestion Possible
1. Can psychological stress interfere with the mutualistic relationship between humans and gut bacteria?
Yes, psychological stress can significantly disrupt the mutualism between humans and their gut microbiota. When chronic stress occurs, it alters the hypothalamic-pituitary-adrenal (HPA) axis, which can, in turn, reduce microbial diversity and increase the proliferation of harmful bacteria. These shifts may affect how gut bacteria affect humans in the mutualism relationship by weakening the production of beneficial metabolites such as short-chain fatty acids. Stress also impacts gut motility and permeability, increasing the risk of inflammation and dysbiosis. Therefore, managing stress through techniques like mindfulness and cognitive-behavioral therapy plays an indirect but critical role in preserving what symbiotic relationships do you think make digestion possible.
2. How does exercise influence the symbiotic relationship that supports digestion?
Physical activity is known to enhance microbial diversity and strengthen the gut barrier, which are key elements in supporting symbiosis. Studies show that regular aerobic exercise increases the abundance of bacteria associated with anti-inflammatory effects, including species that aid fiber fermentation and nutrient absorption. In terms of how gut bacteria affect humans in the mutualism relationship, exercise encourages the growth of beneficial microbes that modulate immune response and enhance energy extraction from food. Additionally, active individuals often exhibit a healthier gut-brain axis, which reinforces digestive health and cognitive resilience. Thus, exercise acts as a functional amplifier of the symbiotic partnerships that make digestion not only efficient but sustainable.
3. Are there geographic or cultural variations in symbiotic digestive relationships?
Absolutely—geography and culture heavily shape the composition and function of the human gut microbiome. Diets rich in plant-based fibers, such as those found in traditional African or Mediterranean regions, promote bacteria involved in producing short-chain fatty acids, reinforcing what symbiotic relationships do you think make digestion possible. Meanwhile, Westernized diets high in refined sugar and fat often reduce microbial diversity and compromise mutualistic interactions. Additionally, cultural practices such as fermented food consumption or traditional medicine can support the longevity of beneficial gut flora. These observations highlight how symbiosis is not just biological but also deeply ecological and sociocultural.
4. Can gut symbiosis influence how medications are metabolized?
Yes, the gut microbiome plays an increasingly recognized role in pharmacokinetics—the movement of drugs through the body. Certain microbial species possess enzymes that can activate or deactivate medications, meaning that what symbiotic relationships do you think make digestion possible may also determine how effective or toxic a drug becomes. This can vary between individuals depending on microbial composition, adding a layer of complexity to personalized medicine. For example, some bacteria can metabolize the cardiac drug digoxin into an inactive form, reducing its therapeutic effects. Understanding these dynamics is crucial for developing precision therapeutics tailored to one’s microbial profile.
5. How does aging impact the mutualistic relationship between humans and their gut bacteria?
Aging tends to reduce microbial diversity and increase the ratio of pro-inflammatory bacterial strains, potentially weakening the mutualism that supports digestion. This shift can impair nutrient absorption, reduce short-chain fatty acid production, and compromise the gut barrier. In terms of how gut bacteria affect humans in the mutualism relationship, these age-related changes may contribute to systemic inflammation, also known as “inflammaging,” and elevate risks for metabolic and neurodegenerative diseases. Diet, exercise, and targeted probiotics can help restore microbial balance in older adults. Maintaining these relationships becomes essential to preserving not only digestive health but also overall vitality and immune resilience in later life.
6. What role do symbiotic microbes play in adapting to dietary changes, such as switching to a high-protein diet?
The microbiome is highly responsive to dietary shifts, often recalibrating its composition within days. When transitioning to a high-protein diet, certain bacterial species that specialize in protein metabolism, such as Bacteroides, tend to proliferate. While this may improve protein digestion, it can also produce harmful metabolites like ammonia or hydrogen sulfide if not balanced with fiber. Thus, understanding what symbiotic relationships do you think make digestion possible is critical when making dietary changes—especially since fiber-loving microbes help neutralize potentially harmful byproducts. A balanced intake of macronutrients supports a more resilient mutualistic environment in the gut.
7. How can urbanization and modern lifestyles threaten gut symbiosis?
Modern lifestyles—characterized by high antibiotic use, reduced fiber intake, and limited exposure to natural environments—can severely compromise the mutualism between humans and gut bacteria. Urban dwellers often have less microbial diversity compared to rural populations, largely due to sanitized environments and processed diets. This reduction undermines how gut bacteria affect humans in the mutualism relationship, as fewer species are available to carry out critical functions like fiber fermentation or vitamin synthesis. The erosion of microbial diversity may also be linked to the rise in autoimmune and allergic diseases in industrialized societies. Promoting biodiversity through diet and nature exposure is essential to preserving the symbiotic relationships that support digestion.
8. Can circadian rhythm disruptions, like jet lag or shift work, affect gut symbiosis?
Yes, circadian misalignment disrupts microbial rhythms and can impair digestive efficiency. The gut microbiome has its own diurnal oscillations, which are closely tied to the host’s sleep-wake cycle. When that cycle is thrown off, microbial populations shift in ways that may reduce metabolic efficiency and increase inflammation. This not only impacts digestion but also influences how gut bacteria affect humans in the mutualism relationship across systems, including immune regulation and hormonal balance. Mitigating circadian disruptions through light therapy, consistent meal timing, and sleep hygiene can help preserve the microbial harmony that underlies digestion.
9. Is there evidence that gut symbiosis can influence food cravings or preferences?
Emerging research suggests that gut microbes may play a role in shaping food preferences by influencing taste receptor expression and neurotransmitter production. Some bacteria appear to promote cravings for the nutrients they thrive on, creating a feedback loop between diet and microbial composition. This opens up fascinating questions about what symbiotic relationships do you think make digestion possible—not just in terms of mechanical processes, but also behavioral influences. For example, increased populations of sugar-loving bacteria may subtly nudge individuals toward sweet foods. Understanding these mechanisms could offer new strategies for managing dietary behaviors and improving nutritional choices.
10. What are some promising future directions in studying gut symbiosis and digestion?
Future research is likely to focus on personalized microbiome modulation, engineered probiotics, and even microbial gene editing. Scientists are beginning to map out which microbial genes are responsible for specific digestive tasks, offering the potential to design bespoke bacterial consortia that optimize how gut bacteria affect humans in the mutualism relationship. We may also see advances in synthetic biology that enable the creation of “designer microbes” capable of targeted drug delivery or toxin neutralization. As we continue to explore what symbiotic relationships do you think make digestion possible, the integration of genomics, artificial intelligence, and systems biology will deepen our ability to fine-tune the gut ecosystem for health optimization.
Reflections on the Microbial Foundations of Digestion and Health
In conclusion, the story of digestion is not solely one of human physiology but of interspecies collaboration. From infancy through old age, humans coexist with trillions of microbial allies who make digestion not only more efficient but more meaningful in the context of overall health. These mutualistic relationships illustrate the biological wisdom of cooperation—of two systems merging into one functional whole. As we deepen our understanding of what symbiotic relationships do you think make digestion possible, we also gain insights into how these same interactions shape our immunity, our cognition, and our emotional well-being. The future of health may well depend on how we nurture these microscopic partnerships, honoring them not as secondary actors but as vital contributors to human life.
Further Reading:
Impacts of Gut Bacteria on Human Health and Diseases
Human gut microbiota in health and disease
Symbiotic Human Gut Bacteria with Variable Metabolic Priorities for Host Mucosal Glycans