The connection between humans and the gut microbiota has captivated the attention of scientists, clinicians, and wellness advocates alike. Once considered merely a passive group of bacteria aiding digestion, the gut microbiota is now recognized as a complex and dynamic ecosystem that plays a central role in human health. From influencing immune responses to regulating mood and cognition, the gut’s microbial residents exert far-reaching effects on both the body and mind. As researchers deepen their understanding of the microbiota and microbiome, it becomes clear that these microscopic communities are anything but incidental. Instead, they are active participants in a symbiotic relationship that shapes our health outcomes across the lifespan.
You may also like: How Gut Health Affects Mental Health: Exploring the Gut-Brain Connection Behind Anxiety, Mood, and Depression
Understanding What Is Microbiota and Why It Matters
To grasp the influence of the gut microbiota, one must first understand what is microbiota and how it differs from the microbiome. The term “microbiota” refers to the trillions of microorganisms—including bacteria, viruses, fungi, and archaea—that inhabit specific environments in and on the human body. When we refer to the gut microbiota, we’re specifically addressing the dense microbial populations residing primarily in the large intestine. The “microbiome,” on the other hand, encompasses the genetic material of all these microorganisms. In essence, microbiota is the cast of characters; the microbiome is their collective script.
The microbiota plays an indispensable role in maintaining homeostasis. It contributes to the digestion of complex carbohydrates, the synthesis of certain vitamins such as B12 and K, and the regulation of immune function. Disruptions in microbial balance—commonly referred to as dysbiosis—can impair these processes, leaving the host vulnerable to a host of chronic conditions, ranging from inflammatory bowel disease to obesity and metabolic syndrome. By understanding what is microbiota in a more nuanced and integrative context, we can begin to appreciate how vital microbial diversity is to our overall well-being.
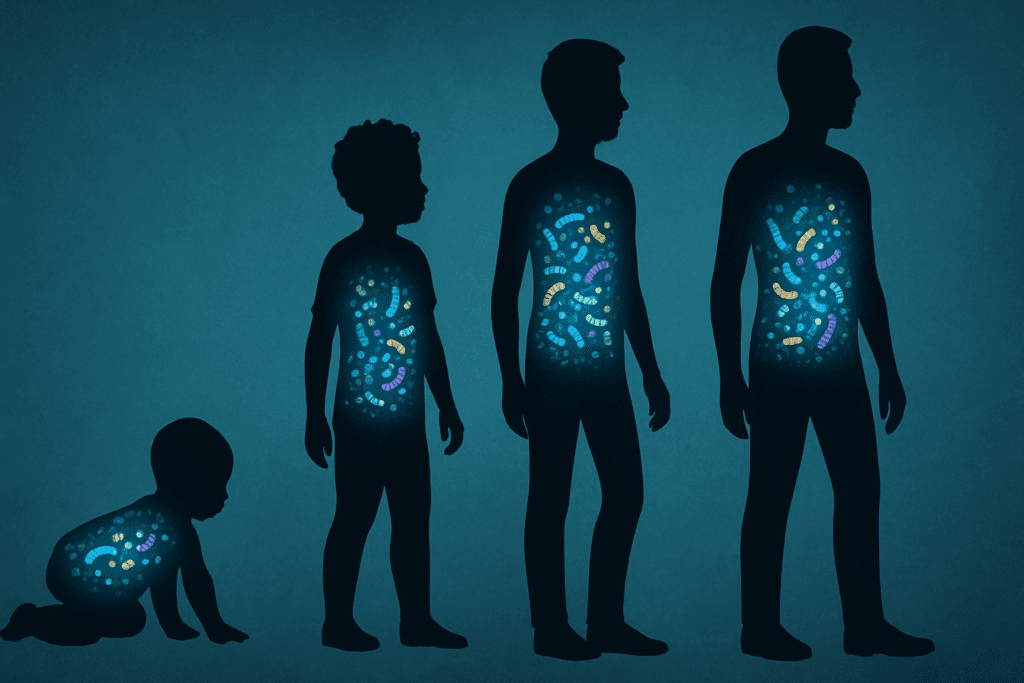
The Development of the Human Microbiota From Birth to Adulthood
The journey of the human gut microbiota begins at birth and evolves through a series of critical developmental stages. At the moment of delivery, newborns are colonized by maternal microbes, a process that varies significantly depending on whether the birth occurs vaginally or via cesarean section. Vaginally delivered infants tend to receive microbial communities resembling those found in the mother’s vaginal canal, while cesarean-born infants acquire microbes more akin to skin flora. This initial seeding has long-term implications for immune development and disease susceptibility.
Breastfeeding further influences the composition of the infant gut microbiota. Human milk contains not only nutrients but also prebiotics and beneficial bacteria such as Bifidobacteria, which support the growth of a healthy microbial community. As solid foods are introduced and children are exposed to new environments, their gut microbiota continues to diversify. By the age of three, the gut microbial ecosystem begins to resemble that of a typical adult, although it remains dynamic and responsive to environmental factors throughout life.
This lifelong adaptability is crucial because humans and the gut microbiota co-evolve in response to changing diets, medications, and stressors. The resilience and plasticity of this ecosystem underscore the importance of early-life interventions, such as breastfeeding and minimizing unnecessary antibiotic use, which can significantly influence health trajectories far into adulthood.
Microbiota and Microbiome in Physical Health and Disease
The impact of the microbiota and microbiome on physical health is both broad and profound. One of the most well-established roles of gut microbes is their influence on metabolic health. Certain microbial species are adept at fermenting dietary fiber into short-chain fatty acids (SCFAs), such as butyrate, which nourish colonocytes and support intestinal integrity. SCFAs also modulate inflammation and influence insulin sensitivity, making them key players in the prevention of metabolic disorders.
Cardiovascular health is another domain increasingly linked to the gut microbiota. For example, the metabolite trimethylamine-N-oxide (TMAO), produced by gut bacteria during the digestion of choline and carnitine-rich foods, has been associated with an elevated risk of atherosclerosis. Conversely, a diverse and balanced microbiota may help lower blood pressure, reduce systemic inflammation, and maintain vascular function.
In addition, emerging evidence connects microbial imbalances with autoimmune and allergic conditions. A lack of microbial diversity has been implicated in the pathogenesis of diseases such as type 1 diabetes, rheumatoid arthritis, and asthma. These findings emphasize that maintaining a healthy microbiota is not merely a matter of digestive comfort but a foundational component of physical health. Understanding the interplay between microbiota and microbiome in various systems of the body opens new avenues for preventative and therapeutic strategies.
The Gut-Brain Axis: A Microbial Link to Mental Health
Perhaps one of the most fascinating developments in recent years is the discovery of the gut-brain axis—a bidirectional communication network linking the central nervous system with the enteric nervous system of the gastrointestinal tract. This axis is modulated in large part by the microbiota. Through the production of neuroactive compounds, modulation of immune function, and direct communication via the vagus nerve, the gut microbiota can influence mood, behavior, and cognitive function.
The implications of this are profound. Numerous studies have shown that alterations in the gut microbiota are associated with mental health conditions such as depression, anxiety, and even neurodevelopmental disorders like autism. Certain strains of Lactobacillus and Bifidobacterium have demonstrated anxiolytic and antidepressant effects in both animal models and human trials, leading to the term “psychobiotics” to describe these beneficial microbes.
Moreover, chronic stress and poor mental health can reciprocally alter the composition of the microbiota, creating a feedback loop that perpetuates dysfunction. Understanding how humans and the gut microbiota interact within this axis could transform the way we approach mental health treatment. Rather than focusing solely on neurochemical imbalances in the brain, clinicians may begin to consider microbial imbalances in the gut as a modifiable risk factor and therapeutic target.

Dietary Patterns That Support a Healthy Microbial Ecosystem
The composition of the gut microbiota is highly sensitive to dietary inputs, and long-term dietary patterns have a more profound effect than short-term changes. Diets rich in plant-based fibers, polyphenols, and fermented foods tend to promote microbial diversity and the proliferation of beneficial species. Mediterranean-style diets, for example, have been shown to increase the abundance of SCFA-producing bacteria and reduce markers of inflammation.
Conversely, Western diets high in processed foods, sugars, and saturated fats can induce dysbiosis. These diets are typically low in fiber and rich in additives that disrupt microbial communities, such as emulsifiers and artificial sweeteners. Over time, this can weaken the gut barrier, increase systemic inflammation, and contribute to metabolic and neurological disorders.
It is important to note that no single diet suits everyone. The concept of personalized nutrition, informed by an individual’s microbiota profile, is gaining traction as a way to optimize both microbial balance and health outcomes. By understanding the connection between microbiota and microbiome and how they respond to different nutrients, we can develop more targeted and sustainable dietary strategies.
The Role of Probiotics and Prebiotics in Microbiota Modulation
In addition to whole foods, targeted supplementation with probiotics and prebiotics offers another avenue for shaping the gut microbiota. Probiotics are live microorganisms that confer health benefits when consumed in adequate amounts. Common strains include Lactobacillus, Bifidobacterium, and Saccharomyces boulardii, each with distinct functions and clinical applications. Prebiotics, on the other hand, are non-digestible fibers that serve as food for beneficial microbes, encouraging their growth and activity.
Clinical studies have demonstrated the efficacy of probiotics in managing a range of conditions, from antibiotic-associated diarrhea to irritable bowel syndrome and certain allergic responses. Prebiotics such as inulin, fructooligosaccharides (FOS), and galactooligosaccharides (GOS) have similarly been shown to enhance microbial diversity and increase SCFA production.
However, not all probiotics are created equal, and their effects can vary depending on the strain, dosage, and host environment. A growing area of research focuses on synbiotics, which combine both probiotics and prebiotics for synergistic effects. As we learn more about what is microbiota and how it influences health, the potential for customized microbial therapies continues to expand.
Environmental and Lifestyle Factors That Shape the Microbiota
While diet is a dominant factor, other aspects of modern life significantly influence the gut microbiota. Antibiotic overuse is a major concern, as it can decimate beneficial microbial populations and pave the way for opportunistic pathogens. Even short courses of antibiotics can cause disruptions that persist for months, underscoring the need for judicious prescribing practices.
Stress, sleep quality, physical activity, and exposure to natural environments also play critical roles. Chronic psychological stress can alter gut permeability and immune responses, creating conditions that favor dysbiosis. Conversely, regular exercise has been associated with increased microbial diversity and the presence of anti-inflammatory species. Spending time outdoors, particularly in green spaces, exposes individuals to a broader range of environmental microbes that can enrich the gut ecosystem.
In urbanized societies, reduced contact with soil, animals, and natural biodiversity has been linked to a rise in autoimmune and allergic disorders. The hygiene hypothesis, and its more refined successor, the “old friends hypothesis,” suggest that early-life microbial exposure is essential for proper immune education. These insights further illustrate the intricate relationship between humans and the gut microbiota and the importance of lifestyle factors in maintaining microbial harmony.
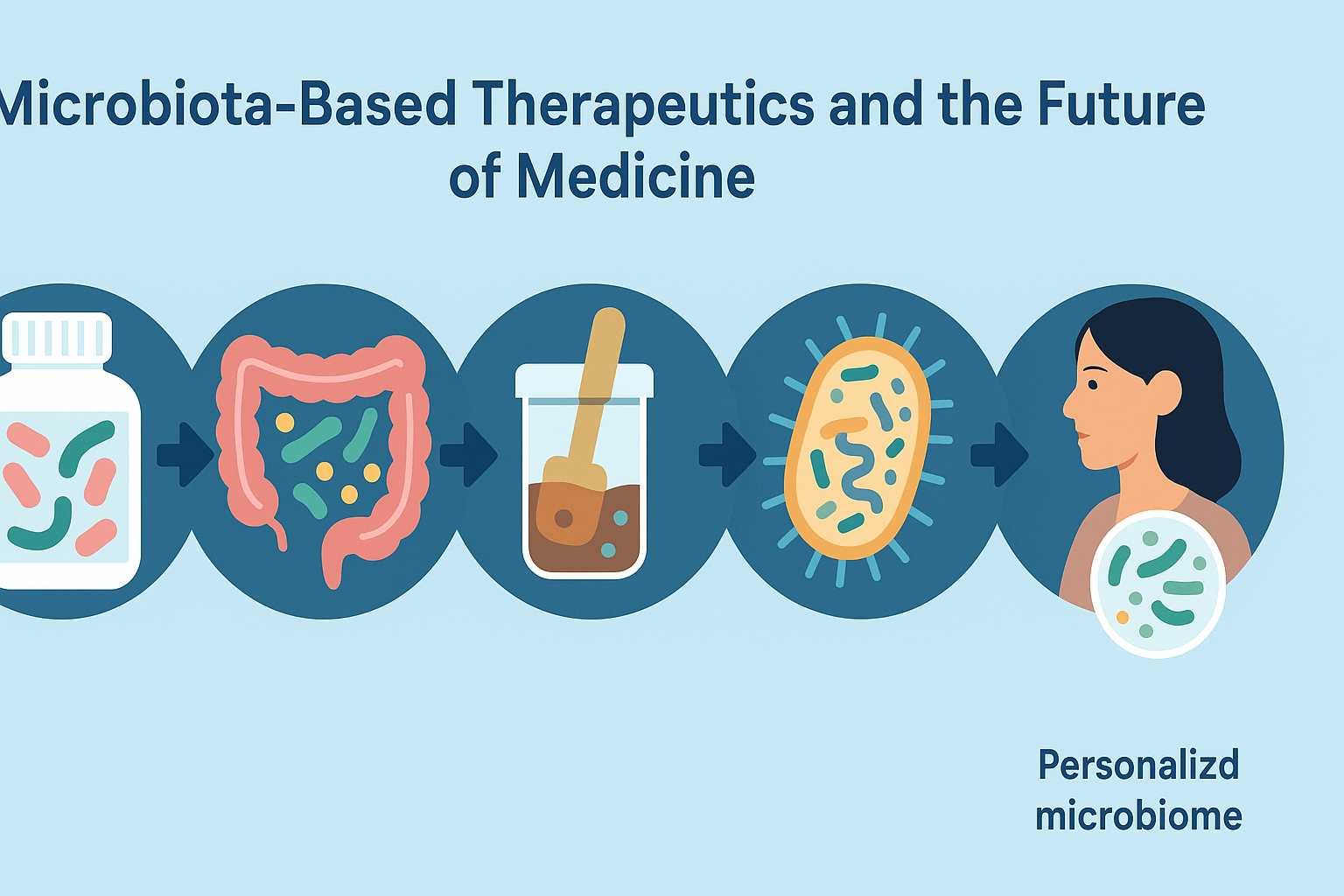
Microbiota-Based Therapeutics and the Future of Medicine
As research into the microbiota and microbiome accelerates, novel therapeutic approaches are beginning to emerge. One of the most promising is fecal microbiota transplantation (FMT), which involves transferring stool from a healthy donor to a patient with a disrupted microbiome. Originally developed to treat recurrent Clostridioides difficile infections, FMT is now being explored for conditions ranging from inflammatory bowel disease to metabolic syndrome and even mood disorders.
Other innovations include microbiota-targeted drugs, designer probiotics, and postbiotics—the bioactive compounds produced by microbes. These therapies aim to manipulate the microbial ecosystem with precision, enhancing beneficial functions while suppressing harmful ones. Advances in sequencing technology and computational modeling have enabled personalized microbiome analysis, paving the way for tailored interventions based on an individual’s unique microbial profile.
The integration of microbiota science into mainstream medicine holds transformative potential. It challenges conventional paradigms by emphasizing the ecosystem within rather than treating isolated symptoms. As our understanding deepens, the question is no longer simply what is microbiota, but how can we work with it to co-create better health.
Frequently Asked Questions: Humans and the Gut Microbiota
1. Can gut microbiota influence how we respond to medications?
Yes, growing evidence suggests that humans and the gut microbiota engage in complex interactions that can significantly influence drug metabolism. Certain bacterial strains are capable of activating, deactivating, or even toxifying medications through enzymatic reactions. For instance, some microbes can alter how the body responds to common drugs like acetaminophen or digoxin. This means that the efficacy and side effects of a medication may depend, in part, on the composition of one’s gut microbiota. Understanding the interplay between microbiota and microbiome in pharmacology is an emerging field known as pharmacomicrobiomics, and it may pave the way for more personalized treatment approaches.
2. How does long-term antibiotic use affect future disease risk?
Long-term antibiotic use has the potential to disrupt the delicate relationship between humans and the gut microbiota, sometimes with lasting consequences. Repeated or prolonged antibiotic exposure can reduce microbial diversity and eliminate beneficial strains that are difficult to replenish naturally. This imbalance has been linked to increased risks of obesity, asthma, allergies, and even colorectal cancer. While antibiotics are crucial for managing infections, their indiscriminate use may cause unintended shifts in the microbiota and microbiome that influence long-term immune regulation. Incorporating post-antibiotic strategies such as prebiotics or targeted probiotics can help restore microbial balance and mitigate potential health risks.
3. Are there gender differences in gut microbiota composition?
Yes, sex-based biological differences extend to the composition and function of the gut microbiota. Hormonal fluctuations related to estrogen and progesterone can influence microbial diversity and metabolic activity, leading to distinctions in how males and females interact with their gut ecosystems. Research suggests that these variations may partly explain why certain autoimmune or metabolic disorders manifest differently between genders. The dialogue between humans and the gut microbiota is shaped not only by genetics and environment but also by hormonal milieus, making sex-specific microbiome research essential for developing tailored therapeutic strategies. Investigating how microbiota and microbiome differences correlate with hormonal cycles and reproductive stages is an area of active study.
4. Can gut microbiota influence sleep and circadian rhythms?
Emerging research indicates that the gut microbiota may play a role in regulating circadian rhythms and sleep quality. Certain microbial species exhibit diurnal patterns and may affect the production of melatonin and serotonin, neurotransmitters essential for sleep regulation. Disruptions in this microbial rhythm, often caused by poor diet, jet lag, or shift work, may contribute to insomnia or other sleep disturbances. Because humans and the gut microbiota maintain a bidirectional feedback loop, changes in sleep-wake cycles can also impact microbial behavior and diversity. This connection offers potential for developing novel treatments for sleep disorders by modulating microbiota and microbiome interactions.
5. How do urban environments impact gut microbial diversity?
Urban living can significantly influence the composition and diversity of gut microbiota due to reduced exposure to natural microbes. Compared to rural populations, individuals in cities often have less contact with soil, animals, and plant life—sources that historically contributed to microbial enrichment. This reduction may partially explain higher rates of allergies, autoimmune disorders, and even mood-related conditions in urban settings. Humans and the gut microbiota have co-evolved with diverse environmental inputs, and modern sanitation, while essential for public health, may inadvertently limit microbial resilience. Encouraging urban dwellers to engage with green spaces or consume a diverse plant-based diet may help compensate for these microbial losses.
6. Is there a link between gut microbiota and food cravings?
Yes, certain microbial populations in the gut may influence host behavior, including food cravings. Some bacteria can produce neurotransmitters or hormonal signals that affect the brain’s reward system, potentially encouraging the host to consume specific nutrients that favor their own survival. For instance, sugar-loving microbes may amplify cravings for high-glycemic foods, subtly shaping dietary choices. This highlights a complex feedback system in which humans and the gut microbiota are not only cohabiting but potentially negotiating over dietary resources. Understanding the influence of microbiota and microbiome dynamics on appetite could lead to more effective interventions for obesity and eating disorders.
7. Can microbiota diversity affect emotional resilience and stress response?
Emotional resilience, or the ability to recover from psychological stress, may be partly shaped by gut microbiota diversity. Individuals with more diverse microbiota tend to show lower cortisol levels and more stable emotional regulation in stressful situations. This may be due to the microbiota’s ability to modulate inflammation and influence neurotransmitter activity, including gamma-aminobutyric acid (GABA) and serotonin. Because the dialogue between humans and the gut microbiota includes immune and neural pathways, maintaining microbial balance may buffer against the physiological toll of chronic stress. Research into resilience-enhancing interventions often now considers the microbiota and microbiome as potential targets for boosting mental well-being.
8. Are there seasonal changes in gut microbiota composition?
Yes, microbiota composition can shift with seasonal changes in diet, activity level, and environmental exposure. Studies in traditional societies and animal models show that variations in food availability—such as increased fiber during harvest months—can lead to predictable microbial fluctuations. Even in industrialized settings, slight changes in microbiota and microbiome profiles have been noted across seasons, likely influenced by diet and lifestyle patterns such as holiday eating or reduced winter exercise. This seasonal adaptability illustrates how humans and the gut microbiota respond dynamically to external stimuli. Maintaining a consistent diet rich in microbial-supportive foods throughout the year can help sustain beneficial microbial populations.
9. How does aging affect the microbiota and its functions?
As people age, the composition and functionality of the gut microbiota often shift in ways that can impact health. There is typically a decline in microbial diversity, accompanied by an increase in pro-inflammatory species. These changes may contribute to age-related conditions such as frailty, cognitive decline, and impaired immune responses. The relationship between humans and the gut microbiota becomes especially significant in older adults, as it may influence everything from nutrient absorption to neurodegeneration. Supporting microbiota and microbiome health through tailored nutrition, prebiotic intake, and physical activity becomes increasingly important as we age.
10. What future technologies could enhance microbiota-based healthcare?
The future of microbiota-based healthcare is promising, with several cutting-edge technologies under development. Precision microbiome mapping, for instance, uses AI and machine learning to create personalized microbial health profiles. This could allow practitioners to recommend individualized diets, probiotics, or even microbial transplants tailored to a person’s unique needs. Additionally, bioengineered probiotics are being designed to deliver specific therapeutic molecules, adding a new layer of functionality to the concept of living medicine. As humans and the gut microbiota continue to be viewed through the lens of systems biology, the role of microbiota and microbiome optimization in preventive and personalized medicine will likely expand dramatically.
Conclusion: Embracing the Microbial Self for Lifelong Health
The relationship between humans and the gut microbiota is a testament to the interconnectedness of life at all scales. Far from being passive inhabitants, the microbes within us are active participants in our physiological and psychological well-being. By understanding what is microbiota and recognizing the complex interplay between microbiota and microbiome, we gain access to a more holistic view of health—one that honors both the scientific intricacies and the practical implications of our microbial partnerships.
This emerging field not only broadens our conception of human biology but also invites a shift in how we care for ourselves. Dietary choices, lifestyle habits, medical treatments, and even our social environments can all be leveraged to support a thriving microbial ecosystem. As the science continues to evolve, embracing this microbial perspective may hold the key to preventing disease, optimizing mental health, and enhancing quality of life across generations. By aligning ourselves with the wisdom of the microbiota, we are not merely treating symptoms; we are nurturing the foundation of human resilience and vitality.
gut health and brain function, digestive wellness tips, probiotics for mental health, gut-brain axis connection, healthy intestinal flora, immune system and gut, fermented foods benefits, microbiome diversity and diet, personalized gut health, chronic inflammation and gut, mental wellness through nutrition, dietary fiber for digestion, SCFA production benefits, natural microbiome support, lifestyle and gut health, antibiotic recovery diet, healthy microbiota balance, enteric nervous system, gut flora and emotions, neurochemical balance and digestion
Was this article helpful? Don’t let it stop with you. Share it right now with someone who needs to see it—whether it’s a friend, a colleague, or your whole network. And if staying ahead on this topic matters to you, subscribe to this publication for the most up-to-date information. You’ll get the latest insights delivered straight to you—no searching, no missing out
Further Reading:
The Role of Gut Microbiota in Anxiety, Depression, and Other
Human gut microbiota in health and disease
How gut bacteria are controlling your brain
Disclaimer
The information contained in this article is provided for general informational purposes only and is not intended to serve as medical, legal, or professional advice. While Health11News strives to present accurate, up-to-date, and reliable content, no warranty or guarantee, expressed or implied, is made regarding the completeness, accuracy, or adequacy of the information provided. Readers are strongly advised to seek the guidance of a qualified healthcare provider or other relevant professionals before acting on any information contained in this article. Health11News, its authors, editors, and contributors expressly disclaim any liability for any damages, losses, or consequences arising directly or indirectly from the use, interpretation, or reliance on any information presented herein. The views and opinions expressed in this article are those of the author(s) and do not necessarily reflect the official policies or positions of Health11News.